Abstract
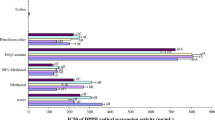
Development of aqueous carotenoid-rich extract (ACE) is a major challenge for the food industry looking for natural colourants. Red capsicum an excellent source of carotenoids has been explored as a novel source for development of ACE through enzymatic liquefaction (EL). Three carbohydrases enzymes viz. viscozyme L, pectinase and cellulase were tested for their liquefaction effects and ability to recover higher carotenoids in aqueous extract. EL significantly (p < 0.05) improved the extract yield and total soluble solids by 2.5-fold to threefold in comparison with unliquefied extract. Incremental increase in dosage of enzymes significantly (p < 0.05) improved the extract yield, total carotenoids, phenolics, ascorbic acid content and antioxidant activity. Viscozyme and pectinase caused significantly higher recovery of carotenoids and other bioactives than cellulase. Viscozyme at dosage of 0.3 % at 60 °C gave the best results. Processing residue or pomace, a spin-off from the EL, was dried to capsicum pomace powder (CPP) and developed as a functional ingredient. The ACE and CPP had higher carotenoid content ranging from 41.72 to 279.83 mg/100 g, respectively. Valorization of capsicum through EL is a promising approach to recover concentrates as valuable food ingredient with reduced processing waste and thus providing sustainability to environment through green processing.








Similar content being viewed by others
References
Abbes F, Kchaoua W, Bleckerb C, Ongenac M, Lognayd G, Attia H, Besbesa S (2013) Effect of processing conditions on phenolic compounds and antioxidant properties of date syrup. Ind Crop Prod 44:634–642
Ahmadi M, Heidari O, Nafchi ARM (2015) Optimization of Lycopene Extraction from Tomato Waste with the Integration of Ultrasonic - Enzymatic Processes by Response Surface Methodology. J. Ind. Eng. Res 1(2):29–34
Alrahmany R, Tsopmo A (2012) Role of carbohydrases on the release of reducing sugar, total phenolics and on antioxidant properties of oat bran. Food Chem 132:413–418
AOAC (1990) Official method of analysis, 15th edn. Association of Official Analytical Chemists, Washington, DC
Apak R, Guclu K, Ozyurek M, Celik SE (2008) Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim Acta 160:413–419
Arnao MB (2000) Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case. Trends Food Sci Technol 11:419–421
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP assay. Anal Biochem 239:70–76
Brand WW, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
Cinar I (2005) Effects of cellulase and pectinase concentrations on the colour yield of enzyme extracted plant carotenoids. Process Biochem 40:945–949
Deepa N, Kaur C, George B, Singh B, Kapoor HC (2007) Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT Food Sci Tecnol 40:121–129
D’Evoli L, Moscatello S, Baldicchi A, Lucarini M, Cruz-Castillo JG, Aguzzi A, Gabrielli P (2007) Post-harvest quality, phytochemicals and antioxidant activity in organic and conventional kiwifruit (Actinidia deliciosa, cv. Hayward). Ital J Food Sci 25(3):362–366
Evans JD, Akin DE, Foulke JA (2002) Flax-retting by polygalacturonase containing enzyme mixtures and effects on fiber properties. J Biotechnol 97:223–231
Haung D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Chem 53:1841–1856
Karadag A, Ozcelik B, Saner S (2009) Review of methods to determine antioxidant capacities. Food Anal Method 2(1):41–60
Kaur C, Walia S, Nangal S, Singh J, Singh BB, Saha S, Singh B, Kalia P, Jaggi S (2013) Functional quality and antioxidant composition of selected tomato (Solanum lycopersicon L.) cultivars grown in Northern India. LWT Food Sci Technol 50:139–145
Khandare V, Walia S, Singh M, Kaur C (2011) Black carrot (Daucus carota ssp. sativus) juice: processing effects on antioxidant composition and color. Food Bioprod Process 89:482–486
Klopotek Y, Otto K, Bohm V (2005) Processing strawberries to different products alters contents of vitamin C, total phenolics, total anthocyanins, and antioxidant capacity. J Agric Food Chem 53:5640–5646
Koley TK, Walia S, Nath P, Awasthi OP, Kaur C (2011) Nutraceutical composition of Zizyphus mauritiana Lamk (Indian ber): effect of enzyme-assisted processing. Int J Food Sci Nutr 62(3):276–279
Kulshreshtha G, Burlot AS, Marty C, Critchle A, Hafting J, Bedoux G, Bourgougnon N, Prithiviraj B (2015) Enzyme-assisted extraction of bioactive material from Chondrus crispus and Codium fragile and its effect on Herpes simplex virus (HSV-1). Mar Drugs 13:558–580
Larrauri JA, Rupérez P, Borroto B, Saura-Calixto F (1996) Mango peels as a new tropical fibre: preparation and characterization. LWT-Food Sci. Technol. 29(8):729–733
Magalhaes LM, Segundo MA, Reis S, Lima JL (2008) Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta 613(1):1–19
Martinez R, Torres P, Meneses AM, Figueroa JG, Pérez-Alvarez GA, Viuda-Martos M (2012) Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem 135(3):1520–1526
Mehrlander K, Dietrich H, Sembries S, Dongowski G, Will F (2002) Structural characterization of oligosaccharides and polysaccharides from apple juices produced by enzymatic pomace liquefaction. J Agric Food Chem 50:1230–1236
Nadeem M, Anjum FM, Khan MR, Saeed M, Riaz A (2011) Antioxidant potential of bell pepper (Capsicum annum L.)—a review. Pak J Food Sci 21(1–4):45–51
Navarro-Gonzalez I, Veronica GV, Javier GA, Jesus PM (2011) Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res Int 44:1528–1535
Oszmianski J, Wojdyło A, Kolniak J (2011) Effect of pectinase treatment on extraction of antioxidant phenols from pomace for the production of puree-enriched cloudy apple juices. Food Chem 127:623–631
Pacheco-palencia A, Lisbeth Hawken P, Talcott ST (2007) Juice matrix composition and ascorbic acid fortification effects on the phytochemical, antioxidant and pigment stability of açai (Euterpe oleracea Mart). Food Chem 105(1):28–35
Pinelo M, Meyer AS (2008) Enzyme-assisted extraction of antioxidants, release of phenols from vegetal matrixes. Electron J Environ Agric Food Chem 7:3217–3220
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53(10):4290–4302
Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing antioxidant power assay. J Agric Food Chem 48:3396
Puri M, Sharma D, Barrow CJ (2011) Enzyme assisted extraction of bioactives from plants. Trends Biotechnol 30(1):37–44
Raghu V, Patel K, Srinivas K (2013) Comparison of ascorbic acid content of Emblica officianalis fruits determined by various analytical methods. J Food Comp Anal 20:529–533
Ramamoorthy K, Bhuvaneswari S, Sankar G, Sakkaravarthi K (2010) Proximate composition and carotenoid content of natural carotenoid sources and its colour enhancement on marine ornamental fish amphiprionocellaris. World J Fish Mar Sci 2(6):545–550
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radical Biol Med 26:1231–1237
Robertson JA, Monredon FD, Dysseler P, Guillon F, Amado R, Thibault TF (2000) Hydration properties of dietary fiber and resistant starch: a European collaborative study. LWT-Food Sci Technol 33:72–79
Rodriguez-Ambriz SL, Islas-Hernandez JJ, Agama-Acevedo E, Tovar J, Bello-Pérez LA (2008) Characterization of a fibre-rich powder prepared by liquefaction of unripe banana flour. Food Chem 107(4):1515–1521
Rombaut N, Tixier AS, Bily A, Chemat F (2014) Green extraction processes of natural products as tools for biorefinery. Biofuels, Bioprod Biorefin 8:530–544
Santamaria RI, Reyes-Duarte RD, Barzana E, Fernando D, Gama FM, Mota M, Lopez-Munguia A (2000) Selective enzyme-mediated extraction of capsaicinoids and caratenoids from chili guajillo puya (Capsicum annum L.) using ethanol as solvent. J Agric Food Chem 48:3063–3067
Singleton VL, Orthofer R, Lamuela-Ranventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteu reagent. Methods Enzymol 299:152–178
Sosulsky FW, Cadden AM (1982) Composition and physiological properties of several sources of dietary fiber. J Food Sci 47:1472–1477
Sowbhagya HB, Chitra VN (2010) Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit Rev Food Sci Nutr 50:146–161
Strati IF, Gogou E, Oreopoulou V (2015) Enzyme and high pressure assisted extraction of carotenoids from tomato waste. J Food Bioprod Proc 94:668–674
Talcott ST, Howard LR, Brenes CH (2000) Antioxidant changes and sensory properties of carrot puree processed with and without periderm tissue. J Agric Food Chem 48:1315–1321
Vega-Galvez A, Lemus Mondaca R, Bilbao-Sainz C, Fito P, Andres A (2008) Effect of air drying temperature on the quality of rehydrated dried red bell pepper (var Lamuyo). J Food Eng 85(1):42–50
Ying SY, Wang Z, Wu J, Chen F, Liao X, Hu X (2006) Optimising enzymatic maceration in pre-treatment of carrot juice concentrate by response surface methodology. Int J Food Sci Technol 41:1082–1089
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Acknowledgments
The authors duly acknowledge Division of Food Science and Postharvest Technology and Division of Agricultural Chemicals, ICAR-Indian Agricultural Research Institute, New Delhi, for providing facilities and financial support during the course of study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nath, P., Kaur, C., Rudra, S.G. et al. Enzyme-Assisted Extraction of Carotenoid-Rich Extract from Red Capsicum (Capsicum annuum). Agric Res 5, 193–204 (2016). https://doi.org/10.1007/s40003-015-0201-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-015-0201-7