Abstract
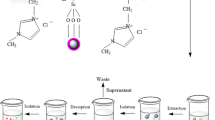
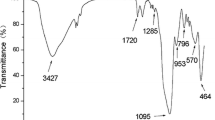
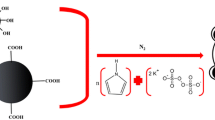
Polyurethane composite adsorbent polymeric material was prepared and investigated for selected solid-phase extraction for metal ions, prior to its determination by inductively coupled plasma optical emission spectrometry. The surface characterisation was done using Fourier transform infrared spectroscopy. The separation and preconcentration conditions of the analytes investigated includes influence of pH, sample loading flow rate, elution flow rate, type and concentration of eluents. The optimum pH for the highest efficient recoveries for all metal ions, which ranged from 70 to 85 %, is pH 7. The metal ions were quantitatively eluted with 5 mL of 2 mol/L HNO3. Common coexisting ions did not interfere with the separation. The percentage recovery of the metal ions ranged between 70 and 89 %, while the results for the limit of detection and limit of quantification ranged from 0.249 to 0.256 and 0.831 to 0.855, respectively. The experimental tests showed good preconcentration results of trace levels of metal ions using synthesised polyurethane polymer adsorbent composite.









Similar content being viewed by others
References
Absalan G, Mehrdjardi MA (2003) Separation and preconcentration of silver ion using 2-mercaptobenzothiazole immobilized on surfactant-coated alumina. Sep Purif Technol 33:95–101
Akl MAA, Kenawy IMM, Lasheen RR (2004) Organically modified silica gel and flame atomic absorption spectrometry: employment for separation and preconcentration of nine trace heavy metals for their determination in natural aqueous systems. Microchem J 78:143–156
Alkan M, Kalay B, Dogan M, Demirbas O (2008) Removal of copper ions from aqueous solutions by kaolinite and batch design. J Hazard Mater 153(1–2):867–876
Anthemidis AN, Themelis DG, Stratis JA (2001) Stopped-flow injection liquid–liquid extraction spectrophotometric determination of palladium in airborne particulate matter and automobile catalysts. Talanta 54:37–43
Anthemidis AN, Zachariadis GA, Stratis JA (2002) Online preconcentration and determination of copper, lead and chromium (VI) using unloaded polyurethane foam packed column by flame atomic absorption spectrometry in natural waters and biological samples. Talanta 58:831–840
Baraka A, Hall PJ, Heslop MJ (2007) Preparation and characterization of melamine formaldehyde–DTPA chelating resin and its use as an adsorbent for heavy metals removal from wastewater. React Funct Polym 67:585–600
Bulut Y, Tez Z (2007) Removal of heavy metals from aqueous solution by sawdust adsorption. J Environ Sci 19(2):160–166
Camel V (2003) Solid phase extraction of trace elements. Spectrochim Acta B At Spectrochim 58:1177–1233
Candir S, Narin I, Soylak M (2008) Ligandless cloud point extraction of Cr(III), Pb(II), Cu(II), Ni(II), Bi(III), and Cd(II) ions in environmental samples with tween 80 and flame atomic absorption spectrometric determination. Talanta 77:289–293
Castro RSD, Caetano L, Ferreira G, Padilha PM, Saeki MJ, Zara LF, Martines MAU, Castro GR (2011) Banana peel applied to the solid phase extraction of copper and lead from river water: preconcentration of metal ions with a fruit waste. Ind Eng Chem Res 50:3446–3451
Chen D, Hu B, Huang C (2009) Chitosan modified ordered mesoporous silica as micro-column packing materials for on-line flow injection-inductively coupled plasma optical emission spectrometry determination of trace heavy metals in environmental water samples. Talanta 78:491–497
Duran A, Tuzen T, Soylak M (2009) Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. J Hazard Mater 169(1–3):466–471
Elci L, Kartal AA, Soylak M (2008) Solid phase extraction method for the determination of iron, lead and chromium by atomic absorption spectrometry using Amberlite XAD-2000 column in various water samples. J Hazard Mater 153(1–2):454–461
El-Sayed GO, Dessouki HA, Ibrahim SS (2010) Biosorption of Ni (II) and Cd (II) ions from aqueous solutions onto rice straw. Chem Sci J 9:1–11
Ensafi AA, Khayamian T, Karbasi MH (2003) Online preconcentration system for lead(II) determination in waste water by atomic absorption spectrometry using active carbon loaded with pyrogallol red. Anal Sci 19:953–956
Ferri T, Sangiorgio P (1996) Determination of selenium speciation in river waters by adsorption of iron(III)-chelex-100 resin and differential pulse cathodic strip** voltammetry. Anal Chimi Acta 321(2–3):185–193
Gayatri SL, Ahmaruzzaman M (2010) Adsorption technique for the removal of phenolic compounds from wastewater using low-cost natural adsorbents. Assam Univ J Sci Technol Phys Sci Technol 5(2):156–166
Gode F, Moral E (2008) Column study on the adsorption of Cr(III) and Cr(VI) using Pumice, YarIkkaya brown coal, Chelex-100 and Lewatit MP 62. Biores Technol 99:1981–1991
Gupta VK, Nayak A (2012) Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J 180:81–90
Gupta VK, Singh P, Rahman N (2004) Adsorption behavior of Hg(II), Pb(II), and Cd(II) from aqueous solution on Duolite C-433: a synthetic resin. J Colloid Interface Sci 275(2):298–402
Gupta VK, Rastogi A, Nayak A (2010) Biosorption of nickel onto treated alga (Oedogonium hatei): application of isotherm and kinetic models. J Colloid Interface Sci 342:533–539
Gupta VK, Agarwal S, Saleh TA (2011a) Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res 45:2207–2212
Gupta VK, Agarwal S, Saleh TA (2011b) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23
Gupta VK, Ganjali MR, Norouzi P, Khani H, Nayak A, Agarawa S (2011c) Electrochemical analysis of some toxic metals by ion-selective electrodes. Critical Rev Anal Chem 41(4):282–313
Gupta VK, Ganjali MR, Nayak A, Bhushan B, Agarwal S (2012a) Enhanced heavy metals removal and recovery by mesoporous adsorbent prepared from waste rubber tire. Chem Eng J 197:330–342
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012b) Chemical treatment technologies for waste-water recycling—an overview RSC. Advances 2(16):6380–6388
Huang X, Chang X, He Q, Cui Y, Zhai Y, Jiang N (2008) Tris(2-Aminoethyl) amine functionalized silica gel for solid-phase extraction and preconcentration of Cr(III), Cd(II) and Pb(II) from waters. J Hazard Mater 157:154
Jain AK, Gupta VK, Bhatnagar A, Suhas A (2003) A comparative study of adsorbents prepared from industrial wastes for removal of dyes. Sep Sci Technol 38(2):463–481
Kiliaris P, Papaspyrides CD (2010) Polymer/layered silicate (clay) nanocomposites: an overview of flame retardancy. Prog Polym Sci 35:902–958
Kocaoba T, Akyuz T (2005) Effects of conditioning of sepiolite prior to cobalt and nickel removal. Desalination 181(1–3):313–318
Krause RWM, Mamba BB, Bambo FM, Malefetse TJ (2010) Cyclodextrin polymers: synthesis and application in water treatment. In: Hu J (ed) Cyclodextrins: chemistry and physics, vol 9. Transworld Research Network, Kerala, pp 185–208
Li A, Zhang Q, Zhang G, Chen J, Fei Z, Liu F (2002) Adsorption of phenolic compounds from aqueous solutions by a water-compatible hypercrosslinked polymeric adsorbent. Chemosphere 47:981–989
Madrakian T, Zolfigol MA, Solgi M (2008) Solid-phase extraction method for preconcentration of trace amounts of some metal ions in environmental samples using silica gel modified by 2,4,6-trimorpholino-1,3,5-triazin. J Hazard Mater 160:468–472
Malla ME, Alvarez MB, Batistoni DA (2002) Evaluation of sorption and desorption characteristics of cadmium, lead and zinc on Amberlite IRC-718 iminodiacetate chelating ion exchanger. Talanta 57:277–287
Mittal A, Mittal J, Malviya A, Gupta VK (2009) Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J Colloid Interface Sci 340(1):16–26
Mittal A, Mittal J, Malviya A, Gupta VK (2010) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interface Sci 344(2):497–507
Mohammadi SZ, Afzali D, Pourtalebi D (2011) Flame atomic absorption spectrometric determination of trace amounts of palladium, gold and nickel after cloud point extraction. J Anal Chem 66(7):620–625
Ngah WS, Teong LC, Toh RH, Hanafiah MAKM (2012) Utilization of chitosan–zeolite composite in the removal of Cu(II) from aqueous solution: adsorption, desorption and fixed bed column studies. Chem Eng J 209:46–53
Olorundare OF, Krause RWM, Okonkwo JO, Mamba BB (2012) Potential application of activated carbon from maize tassel for the removal of heavy metals in water. J Phys Chem Earth 50–52:104–110
Oprea S (2008) Effects of fillers on polyurethane resin-based polyurethane elastomeric bearing materials for passive isolation. J Compos Mater 42(25):2673–2685
Oprea S (2011) Preparation and characterization of the agar/polyurethane composites. J Compos Mater 45(20):2039–2045
Otero-Romani J, Moreda-Pineiro A, Bermejo-Barrera A, Bermejo-Barrera P (2005) Evaluation of commercial C18 cartridges for trace elements solid phase extraction from seawater followed by inductively coupled plasma optical emission spectrometry determination. Anal Chim Acta 536:213–218
Pan L, Qin YR, Hu B, Jiang ZC (2007) Determination of nickel and palladium in environmental samples by low temperature ETV-ICP-OES coupled with liquid- liquid extraction with dimethylglyoxime as both extractant and chemical modifier. Chem Res Chin Univ 23(4):399–403
Pinto ML, Pires J, Carvalho AP, Carvalho MB, Bordado JC (2004) Synthesis and characterization of polyurethane foam matrices for the support of granular adsorbent materials. J Appl Polym Sci 92:2045
Pinto ML, Pires J, Carvalho AP, Carvalho MB, Bordado JC (2005) Characterization of adsorbent materials supported on polyurethane foams by nitrogen and toluene adsorption. Microporous Mesoporous Mater 80:253–262
Rafati L, Mahvi AH, Asgari AR, Hosseini SS (2010) Removal of chromium(VI) from aqueous solutions using Lewatit FO36 nano ion exchange resin. Int J Environ Sci Technol 7(1):147–156
Rezaei B, Sadeghi E, Meghdadi S (2009) Nano-level determination of copper with atomic absorption spectrometry after pre-concentration on N, N-(4-methyl-1, 2-phenylene) diquinoline-2-carboxamide-naphthalene. J Hazard Mater 168(2):787–792
Sabermahani F, Taher MA, Bahrami H, Fozooni S (2011) Alumina coated with oxazolone derivative for extraction of trace amounts of cadmium and copper from water and plant samples. J Hazard Mater 185(2–3):945–950
Sanghavi BJ, Mobin SM, Mathur P, Lahiri GK, Srivastava AK (2013) Biomimetic sensor for certain catecholamines employing copper(II) complex and silver nanoparticle modified glassy carbon paste electrode. Biosens Bioelectron 39:124–132
Sharma RK, Pant P (2009) Pre-concentration and determination of trace metal ions from aqueous samples by newly developed gallic acid modified Amberlite XAD-16 chelating resin. J Hazard Mater 163:295–301
Soylak M, Tuzen M (2008) Coprecipitation of gold(III), palladium(II) and lead(II) for their flame atomic absorption spectrometric determinations. J Hazard Mater 152(2):656–661
Tuzen M, Parlar K, Soylak M (2005) Enrichment/separation of cadmium(II) and lead(II) in environmental samples by solid phase extraction. J Hazard Mater 121:79–87
Zhang L, Chang X, Hu Z, Zhang L, Shi J, Gao R (2010) Selective solid phase extraction and pre-concentration of mercury(II) from environmental and biological samples using nanometer silica functionalized by 2,6-pyridine dicarboxylic acid. Microchim Acta 168:79–85
Acknowledgments
The authors would like to acknowledge the University of Johannesburg for funding (Grant No. 17011 URWC) this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olorundare, O.F., Msagati, T.A.M., Krause, R.W.M. et al. Polyurethane composite adsorbent using solid phase extraction method for preconcentration of metal ion from aqueous solution. Int. J. Environ. Sci. Technol. 12, 2389–2400 (2015). https://doi.org/10.1007/s13762-014-0645-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0645-5