Abstract
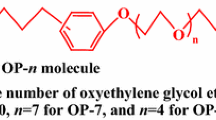
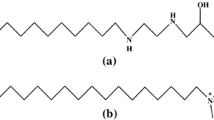
The micellization behavior of amphoteric sodium 3-(N-dodecyl ethylenediamino)-2-hydroxypropyl sulfonate (C12AS) and nonionic octylphenol polyoxyethylene ether having ten oxyethylene glycol ether (OP-10) was investigated in water–isopropanol (IPA)–sodium chloride (NaCl) solution. The critical micelle concentration (cmc) including the single cmc for the single surfactant and the mixed cmc for the binary mixture of C12AS/OP-10 was determined by both tensiometry and the UV–Vis spectrometry. The mole fraction of component (X1 and \(X_{1}^{{{\text{ideal}}}}\)) and activity coefficients (f1 and f2) in mixed micelle, the interaction parameter (β12) between two surfactants, and thermodynamic parameters (ΔGM and (∆Gmic) were estimated according to the pseudophase separation model, the Clint’s model, the Rubingh’s treatment, the Maeda’s treatment, etc. Based on the regular solution theory, cosolvent effects on the interaction between two surfactants were analyzed. It shows that the mole fraction of C12AS in mixed micelle decreases with the concentration of IPA. The addition of IPA in the water/NaCl solution induces a non-monotonous change in the synergistic effect between the two surfactants. Thermodynamic data show that the increase in the concentration of IPA disfavors the spontaneous process of micellization. These phenomena are attributed to the changes in the hydrophobic effect of the hydrophobic group of the surfactant, the electrostatic repulsion between the ionic head groups of C12AS, and the steric effect of the head groups of two surfactants on adding IPA.






Similar content being viewed by others
References
M.J. Rosen, J.T. Junkappu, Surfactants and interfacial phenomena, 4th edn. (Wiley, Hoboken, 2012)
Z.H. Ren, Y. Luo, Tenside Surfact Det. 50, 369–375 (2013)
A. Yousefi, S. Javadian, H. Gharibi, J. Kakemam, M. Rashidi-Alavijeh, J. Phys. Chem. B 115, 8112–8121 (2011)
P.D. Berger, C.H. Berger, Oil recovery method employing amphoteric surfactants, US 7556098, 2009
Z.H. Ren, D.J. Chen, Y. Luo, J. Huang, Acta Chim. Sin. 68, 1771–1775 (2010)
A. Rodriguez, M.M. Graciani, M.L. Moya, Langmuir, 24 (2008) 12785–12792
S. Aslanzadeh, A. Yousefi, J. Surfact Deterg. 17, 709–716 (2014)
M.S. Bakshi, G. Kaur, J. Mol. Liq. 88, 15–32 (2000)
A. Makayssi, R. Bury, C. Treiner, Langmuir, 10 (1994) 1359–1365
Z.H. Ren, J. Ind. Eng. Chem. 20, 3649–3657 (2014)
Z.H. Ren, Ind. Eng. Chem. Res. 53, 10035–10040 (2014)
Z.H. Ren, J. Huang, Y. Lue, Y.C. Zheng, P. Mei, W.C. Yu, L. Lai, Y.L. Chang, F.X. Li, J. Taiwan Inst. Chem. E. 65, 482–487 (2016)
Z.H. Ren, J. Huang, Y. Lue, Y.C. Zheng, P. Mei, W.C. Yu, L. Lai, Y.L. Chang, F.X. Li, Colloids Surf. A Physicochem. Eng. Asp. 504, 131–138 (2016)
Z.H. Ren, J. Huang, Y.C. Zheng, L. Lai, L.L. Hu, J. Mol. Liq. 236, 101–106 (2017)
Z.H. Ren, J. Huang, Y. Lue, Y.C. Zheng, P. Mei, L. Lai, Y.L. Chang, J. Ind. Eng. Chem. 36, 263–270 (2016)
Z.H. Ren, J. Huang, Y.C. Zheng, L. Lai, L.L. Hu, Y.L. Chang, J. Chem. Eng. Data 62, 938–946 (2017)
A. Rodriguez, M.D.M. Graciani, M. Munoz, M.L. Moya, Langmuir, 19 (2003) 7206–7213
P.M. Holland, D.N. Rubingh, J. Phys. Chem. 87, 1984–1990 (1983)
J.H. Clint, J. Chem. Soc. Faraday Trans. 1, 71 (1975) 1327–1334.
P. Molyneux, C.T. Thodes, J. Swarbrich, Trans. Faraday Soc. 61, 1043–1052 (1965)
H. Maeda, J. Colloid Interface Sci. 172, 98–105 (1995)
A.A. Dar, G.M. Rather, S. Ghosh, A.R. Das, J. Colloid Interface Sci. 322, 572–581 (2008)
D. Chandler, Nature 437, 640–647 (2005)
J.A. Long, B.M. Rankin, D. Ben-Amotz, J. Am. Chem. Soc. 137, 10809–10815 (2015)
Z.H. Ren, J. Huang, Y.C. Zheng, L. Lai, P. Mei, X.R. Yu, Y.L. Chang, J. Mol. Liq. 272, 380–386 (2018)
J.E. Gordon, The Organic Chemistry of Electrolyte Solutions (Wiley, New York, 1975)
C.C. Ruiz, J.A. Molina-Bolivar, J. Aguiar, Langmuir 17, 6831–6840 (2001)
D. Maria, R. Amalia, M. Maria, M.M. Luisa, Langmuir 21, 7161–7169 (2005)
S. Puvvada, D. Blankschtein, J. Chem. Phys. 92, 3710–3724 (1990)
Z.H. Ren, Y. Luo, D.P. Shi, Colloids Surf A Physicochem Eng Asp. 428, 18–24 (2013)
S. Javadian, H. Gharibi, Z. Bromand, B. Sohrabi, J. Colloid Interface Sci. 318, 449–456 (2008)
H. Gharibi, S. Javadian, B. Sohrabi, R. Behjatmanesh, J. Colloid Interface Sci. 285, 351–359 (2005)
Acknowledgements
Funding for this work was provided by the National Natural Science Foundation of China (51304029), the Natural Science Foundation of Hubei Province (2016CFB477) and the Hubei Chenguang Talented Youth Development Foundation, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, J., Ren, Z.H. Micellization of binary mixture of amino sulfonate amphoteric surfactant with octylphenol polyoxyethylene ether (10) in water/NaCl solution: effect of isopropanol. J IRAN CHEM SOC 16, 1345–1353 (2019). https://doi.org/10.1007/s13738-019-01607-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01607-4