Abstract
Key message
The soil plant analysis development (SPAD) meter proved effective at estimating leaf Chl content of native and non-native temperate deciduous shrubs. However, due to the change in the relationship between SPAD values and Chl content during the course of autumn senescence, it may be necessary to establish separate calibration equations to cover a range of leaf Chl concentrations.
Context
Non-destructive estimates of leaf chlorophyll (Chl) content by hand-held chlorophyll meters such as the Minolta SPAD-502 provide an effective time-efficient method of collecting field data on senescence.
Aims
To establish useable species-specific calibration equations of relationships between chlorophyll meter readings and leaf Chl content.
Methods
During autumn, we collected SPAD values and quantum yield of photosystem-II (ΦPSII) over a 10-week period from 1044 leaf samples representing native and non-native temperate deciduous shrub species growing in the wild. Subsequently, Chl was extracted from leaf discs (1 cm diameter), incubated at 65 °C for 2 h in dimethyl sulfoxide, and quantified by spectroscopy. Relationships between extracted Chl and SPAD values were established using linear, quadratic, and exponential equations.
Results
Quadratic functions proved the most reliable fit for the data. Interestingly, non-native species tended to have higher leaf Chl content and SPAD values but also exhibited higher variability than native species. The strength (r2) of the SPAD-Chl relationships was weaker than those reported for agricultural or greenhouse-grown plants, but strengthened over time as Chl, ΦPSII, and SPAD values declined.
Conclusion
The SPAD meter proved effective at estimating leaf Chl content of native and non-native temperate deciduous shrubs. These estimates may be used in future to validate remote sensing (satellite and near-surface) vegetation indices in late autumn when trees are leafless.
Similar content being viewed by others
1 Introduction
Despite the pivotal role that shrubs play in temperate deciduous forests by, for example, contributing to carbon and water exchange, nutrient cycling and providing food and habitat for a range of organisms, they have tended to be under-researched relative to trees (Donnelly and Yu 2017, 2019). Shrubs generally leaf out earlier than trees (Harrington et al. 1989; Gill et al. 1998) and non-native shrubs, in particular, often remain photosynthetically active after trees become leafless (Resasco et al. 2007; Wilfong et al. 2009), thus extending the C uptake period of forests. Therefore, the contribution of shrubs to forest productivity and C exchange is particularly important at the extremes of the growing season. Tracking leaf coloration in autumn is often achieved by direct in situ observations (Menzel et al. 2006; Yu et al. 2016; Donnelly et al. 2018) typically of individual forest trees or more recently over much larger geographical scales using satellite-derived phenological metrics extracted from NDVI (normalized difference vegetation index) and EVI (enhanced vegetation index) time series (Zhang and Goldberg 2011; Zhang et al. 2012; Liu et al. 2017). However, an increasing number of reports of discrepancies in the timing of coloration has emerged with the start of satellite-derived leaf color tending to be earlier than direct in situ observations in urban (Donnelly et al. 2018) and forested (Zhao et al. 2020) locations. There are potentially many explanations for these differences, including geographical scale, species composition, and sampling technique. However, another possibility may be that during autumn senescence, chlorophyll (Chl) begins to degrade before visible symptoms are evident but which may be detectable by satellite sensors which are sensitive to small changes in leaf spectral properties. In order to understand the autumn phenology of shrubs under natural conditions, it is necessary to consider different species and in particular to quantify declines in chlorophyll content and variation in autumn senescence between native and non-native shrubs. One way to examine in situ autumn leaf senescence of shrubs is by using a non-destructive chlorophyll meter.
The use of a hand-held chlorophyll meter to estimate foliar Chl content is a cost effective and convenient method of tracking leaf senescence during the autumn season and different meters have been used across a broad range of species (reviewed by Parry et al. 2014 and Table 1). A large number of readings may be achieved in a relatively short period of time without having to remove or destroy leaf tissue for Chl extraction and laboratory analysis. However, in order to ensure accurate and reliable relationships between chlorophyll meter readings and actual leaf Chl content, it is necessary to establish species-specific calibration equations (Markwell et al. 1995; Jifon et al. 2005; Netto et al. 2005; Pinkart et al. 2006; Hawkins et al. 2009; Coste et al. 2010).
Non-destructive optical methods of estimating leaf Chl content using instruments such as either Minolta soil plant analysis development (SPAD) or Opti-Sciences Chlorophyll Content Meter (CCM) have been widely used in agriculture (wheat, rice, cotton, sorghum, soybean, maize, pea, potato, etc.), horticulture (tomato, apple, variety of citrus species, coffee, turfgrass), and viticulture (grapevine) (Table 1). In addition, similar studies have been carried out on (i) temperate tree species either growing in the wild or in plantations (oak, sycamore, maple, birch, beech, eucalyptus), (ii) deciduous shrubs and lianas grown in a common garden, and (iii) a range of tropical and sub-tropical fruit tree species (Table 1). The underlying principle of this method is based on absorption of red light by Chl. The SPAD instrument compares absorbance in the red (~ 650 nm, peak Chl absorbance) and near-infrared (~ 940 nm non-Chl absorbance) parts of the spectrum with the difference being proportional to foliar Chl content (Markwell et al. 1995). The main application of this approach has been in assessing physiological condition, photosynthetic potential, health/nutrient status, senescence rates, and stress levels of target plants for both research and management purposes. Together, these studies clearly demonstrate the reliability of hand-held meters at estimating foliar Chl content for a wide variety of species and applications over a range of environmental conditions. While other leaf pigments such as carotenoids play an important role in autumn senescence, hand-held chlorophyll meters such as SPAD and CCM target wavelengths other than those absorbed by carotenoids and are thus not suitable for examining leaf carotenoids (Peñuelas and Filella 1998). Carotenoid content may show strong relationships with SPAD but only because leaf Chl and carotenoid content are typically highly correlated (e.g., Keskitalo et al. 2005).
Despite the wide range of species for which relationships between non-destructive Chl readings and leaf Chl content have been established (Table 1), including for a number of forest trees, we found only two reports relating to a wetland shrub species (Hawkins et al. 2009) and none relating specifically to temperate deciduous shrubs growing in the wild. In fact, Coste et al. (2010) noted that portable chlorophyll meters were underutilized in forest science while Donnelly and Yu (2017, 2019) reported that shrubs tended to be overlooked in forest research. Therefore, to address this knowledge gap, the aim of the current research was to construct calibration equations to quantify the relationship between chlorophyll meter readings (Minolta SPAD-502) and extracted leaf Chl content for a range of native and non-native temperate deciduous shrub species growing in the wild. We aimed to test two hypotheses (i) the SPAD chlorophyll meter is a robust method to estimate leaf Chl content of temperate deciduous shrubs and (ii) the relationship between SPAD and Chl changes during the autumn senescence process.
2 Materials and methods
2.1 Shrub species, SPAD meter readings, and fluorescence
Leaf color change of eight shrub species representative of the understory layer of a small (11 ha) urban woodland on the campus of the University of Wisconsin-Milwaukee, USA, was monitored during autumn 2018. Shrubs were categorized into native (Ribes americanum Mill., wild currant; Prunus virginiana L., chokecherry; Viburnum acerifolium L., maple leaf viburnum; Viburnum lentago L., nannyberry; Cornus alternifolia L., pagoda dogwood) and non-native (Lonicera morrowii A. Gray, honeysuckle; Rhamnus cathartica L., common buckthorn; Ligustrum vulgare L., European privet) species.
SPAD values (Konica-Minolta, Japan, SPAD-502) and Chl a fluorescence measurements were recorded twice weekly from DOY 250 to 296 on all species and subsequently on two occasions (DOY 302 and 312) when leaves were present only on non-native species. Quantum yield of photosystem II, ΦPSII (Maxwell and Johnson 2000), was measured with a Diving-PAM fluorometer (Walz GmbH, Effeltrich, Germany) following Bott et al. (2008). On each occasion SPAD and ΦPSII readings were recorded at the same location on each of three leaves from three individual plants of each species (nine readings per method per species). All shrubs were shaded by a tall canopy and readings were made between 9:00 and 10:30 am each day, so leaves were exposed only to low light levels and no direct sunlight prior to recording Chl a fluorescence and SPAD values. Diving-PAM excitation light intensity and gain settings were kept constant throughout the whole study to ensure directly comparable Chl a fluorescence values. The same leaves (or leaf part) were subsequently removed and placed in individual labelled plastic bags prior to freezing at − 28 °C for Chl extraction at a later date. All raw data are publically available (Donnelly et al. 2019).
2.2 Chlorophyll extraction
Leaf samples were removed from the freezer and discs, of 1 cm in diameter, were cut from each using a standard borer. Each leaf disc was placed in a 15-ml polyethylene centrifuge tube prior to adding 10 ml dimethyl sulfoxide (DMSO) and the lid secured. The tubes were placed in a water bath and incubated at 65 °C (Hiscox and Israelstam 1979) for 2 h. Preliminary tests indicated that 30 min (Hiscox and Israelstam 1979) or 1 h was insufficient time to render the leaf discs, for all species, colorless. Furthermore, use of DMSO in this study was justified as extracts did not exhibit a brown color like some tree leaf samples reported by Minocha et al. (2009). Samples were removed from the water bath and a 3 ml extract of each supernatant was pipetted individually into a quartz cuvette for spectrophotometer (Beckman-Coulter DU-640) analysis, using a DMSO blank prior to recording absorbance of all leaf samples at 750, 663, 645, and 480 nm.
Total Chl, Chl a, and Chl b concentrations were calculated following the widely (e.g., Richardson et al. 2002; Netto et al. 2005) used equations reported in Arnon (1949), while carotenoid concentration was determined following Wellburn (1994):
Absorbance values were corrected for turbidity using absorbance at 750 nm. Total Chl, Chl a, Chl b, and carotenoid content were calculated on a leaf area basis (mg cm−2 leaf area). All raw data are publically available (Donnelly et al. 2019).
2.3 Temperature data
Temperature (°C) was recorded and logged every 10 min by HOBO sensors at two locations within the study area. Data from the two sensors were averaged and plotted for the duration of the study.
2.4 Outliers, relationship equations, and statistical analysis
On visual inspection of the scatter plots of SPAD vs. total Chl, it was evident that some of the points may have been outliers. In order to statistically determine if any of the points were true outliers, histograms of the residuals of the regression for SPAD and Chl content for each species were constructed and any point that lay outside the normal distribution was considered an outlier and omitted from further analysis (ESMs 1 and 2).
IBM SPSS version 25.0 was used to fit linear, quadratic, and exponential regression equations to the data to explore the relationship between SPAD values and extracted leaf Chl content and to determine which model was the best fit for the data based on r2 and root mean square error (RMSE). MATLAB version 2019a was used to (i) calculate and plot a 15-day moving window of the r2 values for the relationship between SPAD and Chl content to determine the robustness of the relationship over time and (ii) compare changes in parameters over time between native and non-native groups using ANCOVA (analysis of covariance).
3 Results
3.1 Treatment of outliers
Prior to construction of the statistical models, we identified a small number of outliers within the data set. Based on the distribution plots of the residuals, six out of 1044 data points were identified as outliers, and omitted from further analysis as they lay outside the normal distribution of the data set (ESMs 1 and 2). The outliers were for two native (wild currant and dogwood) and one non-native (privet) species and all showed a higher than normal Chl content for a given SPAD value.
3.2 Temperature, leaf fluorescence, and chlorophyll content over time
Temperature (°C) dropped significantly (p < 0.0001) from an average of 15.9 °C (DOY 250–285) to 6.3 °C (DOY 285–312) (Fig. 1a) which was reflected in a marked decline in ΦPSII values for both native and non-native shrubs (Fig. 1b). Interestingly, the temperature drop did not appear to have a detectable impact on the gradual decline in total leaf Chl content (mg cm−2) or SPAD values (Fig. 1c, e). However, the Chl:carotenoid ratio declined over time suggesting that leaf Chl degraded at a faster rate than carotenoids (Fig. 1d).
Site temperature (a), quantum yield of photosystem II ΦPSII (b), total leaf chlorophyll content (c), total leaf chlorophyll:carotenoid ratio (d), and SPAD values (e) during the study period (DOY 250–312 September–November) for 5 native (N = 608 leaves) and 3 non-native (N = 430 leaves) shrub species. Note: scales on x axes differs slightly between (a) and the boxplots
3.3 Leaf chlorophyll content
Total leaf Chl content across the 8 shrub species ranged from 0.0018 to 0.0638 mg cm−2 with native species exhibiting a slightly lower (p < 0.001) content than non-native species (0.0018 to 0.048 mg cm−2 vs. 0.002 to 0.0638 mg cm−2) (Figs. 1c and 2). Similar trends were observed for Chl a and b but a decline in Chl a:Chl b ratio over time indicated faster decline in Chl a than Chl b (ESM 3). The Chl a:Chl b ratio decline was faster in native than non-native species (p < 0.002, ANCOVA). Total leaf Chl content also decreased during autumn senescence though at a similar rate for native and non-native shrub species (Fig. 1c; p = 0.7076 ANCOVA). Carotenoid content also declined as autumn progressed but at a faster rate in native than non-native species (ESM 4; p < 0.002 ANCOVA).
Community (all shrub species (N = 1038) (a), native (N = 608) (b), and non-native (N = 430) (c)) linear (solid line), quadratic (dotted line), and exponential (dashed line) relationships between total leaf chlorophyll content and SPAD meter readings for 5 native and 3 non-native temperate deciduous shrub species. Table 2 presents corresponding conversion equations
3.4 Relationship between SPAD values and leaf chlorophyll content
The scatter plots showed strong positive correlations (p < 0.001) between SPAD values and extracted leaf Chl concentration for (i) the shrub community as a whole, (ii) native and non-native species grou**s, (iii) individual native species, and (iv) individual non-native species (Figs. 2, 3, and 4). The corresponding linear, quadratic, and exponential calibration equations are presented in Tables 2 and 3. Overall, as indicated by relatively high r2 and low RMSE values, the exponential model performed the poorest while the quadratic model performed the best but only marginally better than the linear model. Non-native shrub species showed both the strongest (honeysuckle) and weakest (buckthorn) species-specific correlations, whereas natives exhibited consistently strong relationships (Table 3).
Scatter plots of relationships between total leaf chlorophyll content and SPAD meter readings for 5 native temperate deciduous shrub species. Table 3 presents corresponding linear, quadratic, and exponential calibration equations
Scatter plots of relationships between total leaf chlorophyll content and SPAD meter readings for 3 non-native temperate deciduous shrub species. Table 3 presents corresponding linear, quadratic, and exponential calibration equations
In general, the relationship between SPAD and extracted Chl was weaker at higher values showing higher variability, and this pattern was particularly evident in non-native shrub species (Figs. 2 and 4). Individual species such as buckthorn and privet showed a high degree of scatter throughout the range of Chl contents (Fig. 4).
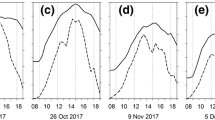
In order to explore how the Chl-SPAD relationship changed as the autumn season progressed, we examined the relationship using a 15-day moving window of the r2 values between SPAD and leaf Chl content (Fig. 5). The r2 values indicated a weak relationship between SPAD and Chl in early-mid autumn which strengthened later in the season when both SPAD and Chl content were low. This pattern was more pronounced in non-native than native species (Fig. 5).
4 Discussion
The majority of studies (Table 1) quantifying the relationship between leaf Chl content and SPAD values focused on a small number of typically agricultural crop species used to inform fertilizer management practices. The only studies reporting multi-species calibration equations were for tropical and subtropical tree species (Schaper and Chacko 1991; Marenco et al. 2009; Coste et al. 2010) while all the temperate deciduous trees for which equations were reported were either grown in a greenhouse (birch Betula papyrifera Richardson et al. (2002)) or under managed conditions (birch Betula pendula Uddling et al. (2007); sycamore Acer pseucoplatanus, oak Quercus robur, beech Fagus sylvatica Percival et al. (2008)). This study and review supports the comment by Coste et al. (2010) that SPAD measurements are underutilized in forest science and particularly in temperate deciduous forests. The current study is the first to report calibration equations (linear, quadratic, and exponential) for leaf Chl content and SPAD values for a range of native and non-native temperate deciduous shrub species growing under natural conditions. As reported in previous studies, albeit on different species, the SPAD meter proved to be a robust method of estimating leaf Chl content of deciduous shrubs in support of our first hypothesis.
A significant shift towards cooler temperatures during the study period (after DOY 285) coincided with a marked decline in ΦPSII coupled with higher variability particularly for native shrubs. Interestingly, the temperature drop had no immediately detectable effect on the declining pigment (Chl or carotenoids) content or SPAD values. This suggests that the photosynthetic apparatus in photosystem II is more sensitive to abrupt decreases in temperature than the total pigment content of a leaf. A similar rapid drop in photosynthetic quantum yield, together with a gradual decline in Chl following a cold shock, was also reported for Aspen (Populus tremula) leaves (Keskitalo et al. 2005). In the current study, as the season progressed, decline in Chl:carotenoid ratios also indicated a slower decline for leaf carotenoid content than for Chl. Chl content degraded at a similar rate for both native and non-native species but carotenoid content decreased at a faster rate in native than non-native shrubs. This retention of carotenoids during loss of Chl has been reported for several species (Lichtenthaler 1987; Keskitalo et al. 2005) and may contribute to extended autumn productivity in non-native shrub species (Harrington et al. 1989; Knight et al. 2007). The range of Chl a:Chl b ratios (~ 1–4.5) in the shrubs was wider than in birch (Richardson et al. 2002), but similar to other deciduous shrubs (Parry et al. 2014). The faster decline in Chl a than Chl b (declining Chl a: Chl b ratio) suggests selective retention of chlorophyll antennae pigments during loss of photosystem chlorophylls as reported for Parthenocissus vine during autumn senescence (Lichtenthaler 1987) and a range of other species (Parry et al. 2014).
In this study we examined the SPAD-Chl relationship over a broad range of values, representing similar ranges reported for tree and other shrub species (e.g., Richardson et al. 2002; Netto et al. 2005; Uddling et al. 2007). Over this broad range, we found that a quadratic fit best described the relationship between total foliar Chl content and SPAD values for a range of temperate deciduous shrubs. Despite finding only marginal differences in the strength of the fit between linear and quadratic relationships, other authors reported that polynomial functions were better at describing the relationship between SPAD values and leaf Chl than linear functions for a range of species including wheat (Monje and Bugbee 1992; Finnan et al. 1997), paper birch (Richardson et al. 2002), coffee (Netto et al. 2005), citrus species (Jifon et al. 2005), spicebush (Hawkins et al. 2009), and Arabidopsis thaliana (Ling et al. 2011). Jifon et al. (2005) proposed that quadratic equations were better than linear models at describing the SPAD-Chl relationship in species with a wide range of chlorophyll concentrations. Furthermore, other authors reported exponential (soybean and maize, Markwell et al. 1995; birch, wheat potato Uddling et al. 2007), linear (grape, Steele et al. 2008), and homographic (neotropical trees, Coste et al. 2010) functions best described the SPAD-Chl relationship. In all the above studies, the reported coefficient of determination (r2) values were consistently above 0.90 compared with the lower r2 values (0.32–0.82) we obtained suggesting that their models were able to explain a greater amount of the variability within the data. Likely reasons for the weaker relationships obtained for our plant species include (i) the longer period over which leaves were sampled, and (ii) the fact that our plants were growing in the wild as opposed to either in a greenhouse or a managed agricultural system.
SPAD values are typically recorded over the course of the senescence period as leaves age but leaves used for calibration determination are generally sampled over a much shorter time period typically on the same day or over a few days to weeks, suggesting that the full range of leaf ages and phenophases may not be attained. However, we took leaf samples for chlorophyll extraction throughout the entire autumn period (10 weeks). Therefore, this study captured a wider range of leaf ages and phenophases which better represents the seasonal change component reflective of a typical SPAD monitoring campaign, especially if applied to phenological or ecological studies. Furthermore, our sample plants were growing in a natural habitat compared with either in controlled environments (e.g., Richardson et al. 2002; Hawkins et al. 2009; Coste et al. 2010; Ling et al. 2011; **ong et al. 2015) or under managed agricultural conditions (e.g., Finnan et al. 1997; Jifon et al. 2005; Netto et al. 2005; Steele et al. 2008) both of which would be expected to result in reduced intraspecific variation. In fact, Jifon et al. (2005) reported that differences in leaf thickness between field- and greenhouse-grown citrus leaves significantly influenced SPAD readings, with thicker field-grown leaves exhibiting higher values for a given Chl content. Therefore, changes in leaf thickness with age, such as leaf thinning as senescence progresses (Ito et al. 2006), may account for some of the variability observed in the relationship between SPAD and leaf Chl content reported here. Furthermore, the senescence period for agricultural crops typically occurs over a shorter period (several weeks) than for temperate deciduous shrubs and trees (several months). Consequently, even though the Chl-SPAD calibration equations reported here were reasonably robust over the study period, it may be useful to establish predictive equations for different phenophases as plants undergo dynamic changes in pigment content and photosynthetic processes over the growth period (Ito et al. 2006).
In agreement with previous reports for birch (Richardson et al. 2002; Uddling et al. 2007), citrus species (orange, lemon, grapefruit) (Jifon et al. 2005), macrolichens (Liu et al. 2019), and agricultural crops (wheat and potato) (Uddling et al. 2007), we found a weaker relationship between SPAD and Chl content at high concentrations of leaf Chl which supports our second hypothesis that the relationship between SPAD and Chl changes over time during autumn senescence and pigment breakdown in leaves. A possible explanation for this change may be due to an uneven distribution of Chl in leaves with high Chl content (Monje and Bugbee 1992; Richardson et al. 2002). This so-called sieve effect (Parry et al. 2014) was more pronounced in non-native than native shrub species. The greater variation in Chl content of non-native species may indicate a greater degree of plasticity for foliar Chl concentration (Harrington et al. 1989). Indeed, Martinez and Fridley (2018) suggested that non-natives may be more plastic than natives in relation to seasonal light changes which may result in more carbon uptake under the same environmental conditions but only if physiological function is maintained (Harrington et al. 1989; Caplan et al. 2018). Even though leaves may be green in late autumn, their photosynthetic capacity is uncertain as components of the photosynthetic apparatus may degrade before total Chl content shows signs of decline (Lichtenthaler 1987; Augspurger et al. 2005; Caplan et al. 2018). These factors may influence interpretation of satellite-derived end of season phenology. For example, commonly used satellite-based phenological metrics, such as NDVI/EVI time series, tend to exhibit an increase in late autumn which is likely related to the extended growing season of shrubs and understory plants after deciduous trees have lost their leaves.
As suggested by previous studies, inter-specific variation in leaf Chl content for a given SPAD value could be as a result of (i) the uneven structure and arrangement of Chl molecules throughout the leaf (Fukshansky et al. 1993; Uddling et al. 2007), (ii) the pattern and density of veins across the leaf surface (McClendon and Fukshansky 1990), (iii) the thickness of the leaf which may change with age (Jifon et al. 2005; Marenco et al. 2009), and (iv) a high rate of chloroplast movement leading to high leaf absorbance changes observed in shade leaves (Davis et al. 2011) all of which may lead to an uneven distribution of Chl within a leaf and may account for at least some of the species-specific differences observed in the current study. Even though we made every effort to avoid veins, especially prominent and mid-rib veins, it is highly likely that at least some leaf samples and SPAD values included a small portion of vein, thus contributing to this observed inter-specific variation.
5 Conclusions
The hand-held SPAD-502 meter produced reliable estimates of leaf Chl content for a range of, native and non-native, temperate deciduous shrubs. The progressively stronger relationship between SPAD values and Chl content during the course of autumn senescence as Chl degraded suggests the need for establishing calibration equations to cover a range of leaf Chl concentrations if absolute Chl content is required. Overall, coefficients of determination were higher for quadratic functions and for the native shrub community compared with the non-native community indicating a stronger relationship between SPAD and leaf Chl content. Given the interspecific variation observed in this study, we recommend deriving species-specific calibration equations for different shrub species to determine the relationship between SPAD and Chl content. Nonetheless, SPAD can provide a useful means of comparing relative Chl content if required. In future, we anticipate exploring the potential use of SPAD values to examine the, often observed, peak in remote sensing vegetation indices in late autumn when upper canopy trees are leafless.
Data availability
The raw data used in this paper has been accepted by the data repository service PANGAEA but will not be released until our paper has been published. To view the dataset go to the url below and search for Alison Donnelly. This should bring you to following dataset: Donnelly A, Yu R, Rehberg C, Meyer G, Young EB (2019) Shrub leaf chlorophyll content and SPAD values. Extracted leaf chlorophyll content with corresponding SPAD values of temperate deciduous native and non-native shrubs, autumn 2018, southern Wisconsin, USA. https://doi.org/10.1594/PANGAEA.907932
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Augspurger CK, Cheesman JM, Salk CF (2005) Light gains and physiological capacity of understory woody plants during phenological avoidance of canopy shade. Funct Ecol 19:537–546
Bielinis E, Jóźwiak W, Robakowski P (2015) Modelling the relationship between the SPAD values and photosynthetic pigments content in Quercus petraea and Prunus serotine leaves. Dendrobiol 73:125–134
Bindi M, Hacour A, Vandermeiren K, Craigon J, Ojanpera K, Selldén T, Högy P, Finnan J, Fibbi L (2002) Chlorophyll concentration of potatoes grown under elevated carbon dioxide and/or ozone concentrations. Eur J Agron 17:319–335
Bott T, Meyer GA, Young EB (2008) Nutrient limitation and morphological plasticity of the carnivorous pitcher plant Sarracenia purpurea in contrasting wetland environments. New Phytol 180:631–641
Campbell RJ, Mobley KN, Marini RP, Pfeiffer DG (1990) Growing conditions alter the relationship between SPAD-501 values and apple leaf chlorophyll. Hortscience 25:330–331
Caplan JS, Whitehead RD, Gover AE, Grabosky JC (2018) Extended leaf phenology presents an opportunity for herbicidal control of invasive forest shrubs. Weed Res 58:244–249
Castelli F, Contillo R, Miceli F (1996) Non-destructive determination of leaf chlorophyll content in four crop species. J Agron Crop Sci 177:275–283
Coste S, Baraloto C, Leroy C, Marcon E, Renaud A, Richardson A, Roggy J-C, Schimann H, Uddling J, Hérault B (2010) Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann For Sci 67:607
Davis PA, Vaylor S, Whippo CW, Hangarter RP (2011) Changes in leaf optical properties associated with light dependent chloroplast movement. Plant Cell Environ 34:2047–2059
Donnelly A, Yu R (2017) The rise of phenology with climate change: an evaluation of IJB publications. Int J Biometerol 61:29–50
Donnelly A, Yu R (2019) Temperate deciduous shrub phenology: the overlooked forest layer. Int J Biometerol:1–13. https://doi.org/10.1007/s00484-019-01743-9
Donnelly A, Jones MB, Burke JI, Schnieders B (2000) Elevated CO2 provides protection from O3 induced photosynthetic damage and chlorophyll loss in flag leaves of spring wheat (Triticum aestivum L. c.v. Minaret). Agric Ecosyst Environ 80:159–168
Donnelly A, Craigon J, Black CR, Colls JJ, Landon G (2001) Does elevated CO2 ameliorate the impact of O3 on chlorophyll content and photosynthesis in potato (Solanum tuberosum)? Physiol Plant 111:501–511
Donnelly A, Liu L, Zhang X, Wingler A (2018) Autumn leaf phenology: discrepancies between in situ observations and satellite data at urban and rural sites. Int J Remote Sens. https://doi.org/10.1080/01431161.2018.1482021
Donnelly A, Yu R, Rehberg C, Meyer G, Young EB (2019) Extracted leaf chlorophyll content with corresponding SPAD values of temperate deciduous native and non-native shrubs, autumn 2018, southern Wisconsin, USA. PANGAEA, https://doi.org/10.1594/PANGAEA.907932
Dwyer LM, Tolenaar M, Houwing L (1991) A non-destructive method to monitor leaf greenness in corn. Can J Plant Sci 71:505–509
Fanizza G, Della Gatta C, Bagnulo C (1991) A non-destructive determination of leaf chlorophyll in Vitis vinifera. Ann Appl Biol 119:203–205
Finnan JM, Burke JI, Jones MB (1997) A note on a non-destructive method of chlorophyll determination in wheat (Triticum aestivum L.). Irish J Agric Food Res 36:85–89
Fridley JD (2012) Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 485:359–362
Fukshansky L, Martinex A, Remisowsky V, McClendon J, Ritterhusch A, Richter T, Mohr H (1993) Absorption spectra of leaves corrected for scatter and distributional error: a radiative transfer and absorption statistics treatment. Photochem Photobiol 57:538–535
Gill DS, Amthor JS, Bormann FH (1998) Leaf phenology, photosynthesis, and the persistence of saplings and shrubs in a mature northern hardwood forest. Tree Physiol 18:281–289
Harrington RA, Brown BJ, Reich PB (1989) Ecophysiology of exotic and native shrubs in Southern Wisconsin. I. Relationship of leaf characteristics, resource availability, and phenology to seasonal patterns of carbon gain. Oecologia 80:356–367
Hawkins TS, Gardiner ES, Comer GS (2009) Modeling the relationship between extractable chlorophyll and SPAD-502 readings for endangered plant species research. J Nat Conserv 17:125–129
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Ito A, Muraoka H, Koisumi H, Saigusu N, Murayama S, Yamamoto S (2006) Seasonal variation in leaf properties and ecosystem carbon budget in a cool-temperate deciduous broad-leaved forest: simulation analysis at Takayama site, Japan. Ecol Res 21:137–149
Jiang XX, Vergara BS (1986) Chlorophyll meter to quantify relative cold tolerance in rice. Internat Rice Res Newsletter 11:10–11
Jifon JL, Syvertsen JP, Whaley E (2005) Growth environment and leaf anatomy affect nondestructive estimates of chlorophyll and nitrogen in Citrus sp. leaves. J Am Soc Hortic Sci 130:152–158
Kapotis G, Zervoudakis G, Veltsistas T, Salahas G (2003) Comparison of chlorophyll meter readings with leaf chlorophyll concentration in Amaranthus vlitus: correlation with physiological processes. Russ J Plant Physiol 50:395–397
Keskitalo F, Bergquist G, Gardeström P, Jansson S (2005) A cellular timetable of autumn senescence. Plant Physiol 139:1635–1648
Knight KS, Kurylo JS, Endress AG, Stewart JR, Reich SB (2007) Ecology and ecosystem impacts of common buckthorn (Rhamnus cathartica): a review. Biol Invasions 9:925–937
Lichtenthaler HK (1987) Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J Plant Physiol 131:101–110
Ling Q, Huang W, Jarvis P (2011) Use of a SPAD-520 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth Res 107:209–214
Liu L, Zhang X, Yu Y, Donnelly A (2017) Detecting spatiotemporal changes of peak foliage coloration in deciduous and mixed forests in the Central and Eastern United States. Environ Res Lett 12:024013
Liu S, Li S, Fan XY, Yuan GD, Hu T, Shi XM, Huang JB, Pu XY, Wu CS (2019) Comparison of two noninvasive methods for measuring the pigment content in foliose macrolichens. Photosynth Res 141:245–257
Marenco RA, Antezana-Vera SA, Nascimento HCS (2009) Relationship between specific leaf area, leaf thickness, leaf water content and SPAD-502 readings in six Amazonian tree species. Photosynth 47:184–190
Markwell J, Osterman JC, Mitchell JL (1995) Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth Res 46:467–472
Marquard RD, Tipton JL (1987) Relationship between extractable chlorophyll and in situ method to estimate leaf greenness. Hortscience 22:1327
Martinez KA, Fridley JD (2018) Acclimation of leaf traits in seasonal light environments: are non-native species more plastic? J Ecol 106:2019–2030
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 51:659–668
McClendon JH, Fukshansky L (1990) On the interpretation of absorption spectra of leaves 1. The introduction and the correction of leaf spectra for surface reflection. Photochem Photobiol 51:203–210
Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, Braslavská O, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl Å, Defila C, Donnelly A, Filella Y, Jatczak K, Måge F, Mestre A, Nordli Ø, Peñuelas J, Pirinen P, Remisová V, Scheifinger H, Striz M, Susnik A, Wielgolaski F-E, van Vliet A, Zach S, Zust A (2006) European phenological response to climate change matches the warming pattern. Glob Chang Biol 12:1–8
Minocha R, Martinez G, Lyons B, Long S (2009) Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can J For Res 39:849–861
Monje OA, Bugbee B (1992) Inherent limitations of non-destructive chlorophyll meters: a comparison of two types of meters. Hortscience 27:69–71
Nauš J, Prokopová J, Řebíček J, Špundová M (2010) SPAD chlorophyll meter reading can be pronouncedly affected by chloroplast movement. Photosynth Res 105:265–271
Netto AT, Campostrini E, Gonçlaves de Oliveira J, Bressan-Smith RE (2005) Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci Hortic 104:199–209
Parry C, Blonquist JM Jr, Bugbee B (2014) In situ measurement of leaf chlorophyll concentration: analysis of the optical/absolute relationship. Plant Cell Environ 37:2508–2520
Peñuelas J, Filella I (1998) Visible and near0infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci 3:151–156
Percival GC, Keary IP, Noviss K (2008) The potential of a chlorophyll content SPAD meter to quantify nutrient stress in foliar tissue of sycamore (Acer pseudoplatanus), English Oak (Quercus robur) and European Beech (Fagus sylvatica). Arboricult Urban For 34:89–100
Piekkielek WP, Fox RH (1992) Use of a chlorophyll meter to predict sidedress nitrogen. Agron J 84:59–65
Pinkart EA, Patel V, Mohammed C (2006) Chlorophyll and nitrogen determination for plantation-grown Eucalyptus nitens and E. globulus using a non-destructive meter. For Ecol Manag 223:211–217
Resasco J, Hale AN, Henry MC, Gorchov DL (2007) Detecting an invasive shrub in a deciduous forest understory using late-fall Landsat sensor imagery. Int J Remote Sens 28:3739–3745
Richardson AD, Duigan S, Berlyn G (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol 153:185–194
Rodriguez IR, Miller GL (2000) Using a chlorophyll meter to determine the chlorophyll concentration, nitrogen concentration and visual quality of St. Augustinegrass. Hortscience 35:751–754
Schaper H, Chacko EK (1991) Relationship between extractable chlorophyll and portable chlorophyll meter readings in leaves of eight tropical and subtropical fruit-tree species. J Plant Physiol 138:674–677
Sibley JL, Eakes DJ, Gilliam CH, Keever GJ, Dozier WA Jr, Himelrick DG (1996) Foliar SPAD-502 meter values, nitrogen levels, and extractable chlorophyll for red maple selections. Hortscience 31:468–470
Steele M, Gitelson AA, Rundquist D (2008) Nondestructive estimation of leaf chlorophyll content in grapes. Pap Nat Res 282 (http://digitalcommonsunledu/natrespapers/282)
Tenga AZ, Marie BA, Ormrod DP (1989) Leaf greenness meter to assess ozone injury to tomato leaves. Hortscience 24:514
Uddling J, Gelang-Alfredsson J, Piikki K, Pleijel H (2007) Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth Res 91:37–46
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids; using various solvents and spectrophotometers of different resolution. J Plant Physiol 144:307–303
Wilfong BN, Gorchov DL, Henry MC (2009) Detecting an invasive shrub in deciduous forest understories using remote sensing. Weed Sci 57:512–520
**ong D, Chen J, Yu T, Gao W, Ling X, Li Y, Peng S, Huang J (2015) SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci Rep-UK 5:13389. https://doi.org/10.1038/srep13389
Xu W, Rosenow DT, Nguyen HT (2000) Stay green trait in grain sorghum: relationship between visual rating and leaf chlorophyll concentration. Plant Breed 119:362–367
Yamamoto A, Nakamura T, Adu-Gyamfi JJ, Saigusa M (2002) Relationship between chlorophyll content in leaves of sorghum and pigeonpea determined by extraction method and by chlorophyll meter (SPAD-502). J Plant Nutr 25:2295–2301
Yu R, Schwartz MD, Donnelly A, Liang L (2016) Modeling the progression of spring and autumn phenology of deciduous trees. Int J Biometerol 59:1–15
Zhang X, Goldberg MD (2011) Monitoring fall foliage coloration dynamics using time-series satellite data. Remote Sens Environ 115:382–391
Zhang XY, Goldberg MD, Yu YY (2012) Prototype for monitoring and forecasting fall foliate coloration in real time from satellite data. Agric For Meteorol 158:21–29
Zhao B, Donnelly A, Schwartz M (2020) Evaluating autumn phenology derived from field observations, satellite data, and carbon flux measurements in a northern mixed forest, USA. Int J Biometerol:1–15. https://doi.org/10.1007/s00484-020-01861-9
Acknowledgments
We thank John Berges for help with leaf sampling and Mark Schwartz for making temperature data available. We are grateful to Gretchen Meyer (UWM Field Station director) for granting us access to Downer Woods, help with species identification, and for useful comments on the manuscript.
Funding
The authors would like to acknowledge support from the University of Wisconsin-Milwaukee Research Growth Initiative grant number 101x368.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest
Additional information
Handling Editor: Erwin Dreyer
This paper is dedicated to the memory of John Finnan, an outstanding agronomist.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contribution of the co-authors
AD conceived of idea, designed experiment, collected data, performed chlorophyll extractions, carried out data analysis, and wrote manuscript.
EBY collected data, defined chlorophyll extraction method, and commented on data analysis and manuscript in general.
GM confirmed shrub identification and commented on data analysis and manuscript in general.
CR helped with data collection and chlorophyll extractions and commented on manuscript.
RY carried out statistical tests, advised on data analysis, and commented on manuscript in general.
Electronic supplementary material
ESM 1
(DOCX 131 kb).
Rights and permissions
About this article
Cite this article
Donnelly, A., Yu, R., Rehberg, C. et al. Leaf chlorophyll estimates of temperate deciduous shrubs during autumn senescence using a SPAD-502 meter and calibration with extracted chlorophyll. Annals of Forest Science 77, 30 (2020). https://doi.org/10.1007/s13595-020-00940-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-020-00940-6