Abstract
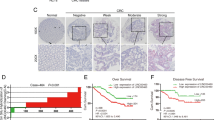
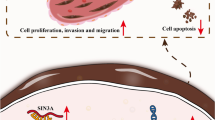
The long noncoding RNA (lncRNA) LINC00520 is an important modulator of the oncogenicity of multiple human cancers. However, whether LINC00520 is involved in the malignant characteristics of colorectal cancer (CRC) has not been extensively studied until recently. Therefore, the present study aimed to detect LINC00520 expression in CRC and evaluate its clinical significance in patients with CRC. Functional experiments were conducted to test the biological roles and underlying mechanisms of LINC00520 in CRC progression. In this study, high-LINC00520 expression was verified in CRC tissues and cell lines, and this high expression was associated with patients’ unfavorable clinicopathological parameters and shorter overall survival and disease-free survival. Functionally, interference of LINC00520 resulted in a significant decrease of CRC cell proliferation, migration, colony forming ability, and invasion. Mechanistically, LINC00520 functioned as a competing endogenous RNA by sponging microRNA-577 (miR-577) and thereby increasing heat shock protein 27 (HSP27) expression. Rescue experiments revealed that inhibiting miR-577 or restoring HSP27 could abrogate the effects of LINC00520 silencing on malignant phenotypes of CRC. LINC00520 functioned as an oncogenic lncRNA in CRC, and it facilitated CRC progression by regulating the miR-577/HSP27 axis, suggesting that the LINC00520/miR-577/HSP27 axis is an effective target in anticancer management.






Similar content being viewed by others
References
Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. https://doi.org/10.3322/caac.21220.
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. https://doi.org/10.1016/S0140-6736(13)61649-9.
Welch HG, Robertson DJ. Colorectal cancer on the decline—why screening can't explain it all. N Engl J Med. 2016;374:1605–7. https://doi.org/10.1056/NEJMp1600448.
Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11:835–48. https://doi.org/10.1038/nrc3162.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Guo P, Huang ZL, Yu P, Li K. Trends in cancer mortality in China: an update. Ann Oncol. 2012;23:2755–62. https://doi.org/10.1093/annonc/mds069.
Lv Y, Huang S. Role of non-coding RNA in pancreatic cancer. Oncol Lett. 2019;18:3963–73. https://doi.org/10.3892/ol.2019.10758.
Chi Y, Wang D, Wang J, Yu W, Yang J. Long non-coding RNA in the pathogenesis of cancers. Cells. 2019. https://doi.org/10.3390/cells8091015.
Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9:1354–66.
Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–307. https://doi.org/10.1016/j.cell.2013.02.012.
Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41. https://doi.org/10.1016/j.cell.2009.02.006.
Fernandes JC, Acuna SM, Aoki JI, Floeter-Winter LM, Muxel SM. Long non-coding RNAs in the regulation of gene expression: physiology and disease. Noncoding RNA. 2019. https://doi.org/10.3390/ncrna5010017.
Hauptman N, Glavac D. Long non-coding RNA in cancer. Int J Mol Sci. 2013;14:4655–69. https://doi.org/10.3390/ijms14034655.
Bao Z, Yang Z, Huang Z, Zhou Y, Cui Q, Dong D. LncRNADisease 2.0: an updated database of long non-coding RNA-associated diseases. Nucleic Acids Res. 2019;47:D1034–D10371037. https://doi.org/10.1093/nar/gky905.
Liu W, Wang Z, Wang C, Ai Z. Long non-coding RNA MIAT promotes papillary thyroid cancer progression through upregulating LASP1. Cancer Cell Int. 2019;19:194. https://doi.org/10.1186/s12935-019-0913-z.
Zhuang X, Tong H, Ding Y, Wu L, Cai J, Si Y, Zhang H, Shen M. Long noncoding RNA ABHD11-AS1 functions as a competing endogenous RNA to regulate papillary thyroid cancer progression by miR-199a-5p/SLC1A5 axis. Cell Death Dis. 2019;10:620. https://doi.org/10.1038/s41419-019-1850-4.
Chen ZB, Cao WL, Su K, Mao M, Zeng XY, Li JH. MIR22HG inhibits cell growth, migration and invasion through regulating the miR-24-3p/p27kip1 axis in thyroid papillary carcinomas. Eur Rev Med Pharmacol Sci. 2019;23:5851–62. https://doi.org/10.26355/eurrev_201907_18327.
**a F, Chen Y, Jiang B, Du X, Peng Y, Wang W, Huang W, Feng T, Li X. Long noncoding RNA HOXA-AS2 promotes papillary thyroid cancer progression by regulating miR-520c-3p/S100A4 pathway. Cell Physiol Biochem. 2018;50:1659–72. https://doi.org/10.1159/000494786.
Wu DM, Wang S, Wen X, Han XR, Wang YJ, Shen M, Fan SH, Zhang ZF, Shan Q, Li MQ, Hu B, Lu J, Chen GQ, Zheng YL. LncRNA SNHG15 acts as a ceRNA to regulate YAP1-Hippo signaling pathway by sponging miR-200a-3p in papillary thyroid carcinoma. Cell Death Dis. 2018;9:947. https://doi.org/10.1038/s41419-018-0975-1.
Xu Y, Wang J, Wang J. Long noncoding RNA XIST promotes proliferation and invasion by targeting miR-141 in papillary thyroid carcinoma. OncoTargets Ther. 2018;11:5035–43. https://doi.org/10.2147/OTT.S170439.
Wu YY, Gao W, Zhang YL, Niu M, Cui JJ, **ang CX, Sang JW, Wen SX, Wang BQ. Expression and clinical significance of long non-coding RNA LINC00520 in laryngeal squamous cell carcinoma. Lin Chung Er Bi Yan Hou Tou **g Wai Ke Za Zhi. 2018;32:91–5. https://doi.org/10.13201/j.issn.1001-1781.2018.02.003.
**e T, Pi G, Yang B, Ren H, Yu J, Ren Q, Zhou X, Hu D, Zhang H, Zhang H, Zhang Q, Hu L, Li Y, Zhou F. Long non-coding RNA 520 is a negative prognostic biomarker and exhibits pro-oncogenic function in nasopharyngeal carcinoma carcinogenesis through regulation of miR-26b-3p/USP39 axis. Gene. 2019;707:44–52. https://doi.org/10.1016/j.gene.2019.02.093.
Henry WS, Hendrickson DG, Beca F, Glass B, Lindahl-Allen M, He L, Ji Z, Struhl K, Beck AH, Rinn JL, Toker A. LINC00520 is induced by Src, STAT3, and PI3K and plays a functional role in breast cancer. Oncotarget. 2016;7:81981–94. https://doi.org/10.18632/oncotarget.11962.
Mei XL, Zhong S. Long noncoding RNA LINC00520 prevents the progression of cutaneous squamous cell carcinoma through the inactivation of the PI3K/Akt signaling pathway by downregulating EGFR. Chin Med J (Engl). 2019;132:454–65. https://doi.org/10.1097/CM9.0000000000000070.
Jiang H, Ju H, Zhang L, Lu H, Jie K. MicroRNA-577 suppresses tumor growth and enhances chemosensitivity in colorectal cancer. J Biochem Mol Toxicol. 2017. https://doi.org/10.1002/jbt.21888.
Wang Y, Lu Z, Wang N, Feng J, Zhang J, Luan L, Zhao W, Zeng X. Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp Mol Med. 2018;50:57. https://doi.org/10.1038/s12276-018-0082-5.
Chu F, Xue L, Miao H. Long noncoding RNA TP73-AS1 in human cancers. Clin Chim Acta. 2020;500:104–8. https://doi.org/10.1016/j.cca.2019.09.024.
Gourvest M, Brousset P, Bousquet M. Long noncoding RNAs in acute myeloid leukemia: functional characterization and clinical relevance. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11111638.
Ma S, Long T, Huang WJ. Noncoding RNAs in inflammation and colorectal cancer. RNA Biol. 2019. https://doi.org/10.1080/15476286.2019.1705610.
Abdollahzadeh R, Daraei A, Mansoori Y, Sepahvand M, Amoli MM, Tavakkoly-Bazzaz J. Competing endogenous RNA (ceRNA) cross talk and language in ceRNA regulatory networks: a new look at hallmarks of breast cancer. J Cell Physiol. 2019;234:10080–10000. https://doi.org/10.1002/jcp.27941.
McConnell JR, McAlpine SR. Heat shock proteins 27, 40, and 70 as combinational and dual therapeutic cancer targets. Bioorg Med Chem Lett. 2013;23:1923–8. https://doi.org/10.1016/j.bmcl.2013.02.014.
Yuan W, Sui C, Liu Q, Tang W, An H, Ma J. Up-regulation of microRNA-145 associates with lymph node metastasis in colorectal cancer. PLoS ONE. 2014;9:e102017. https://doi.org/10.1371/journal.pone.0102017.
Hung CS, Huang CY, Lee CH, Chen WY, Huang MT, Wei PL, Chang YJ. IGFBP2 plays an important role in heat shock protein 27-mediated cancer progression and metastasis. Oncotarget. 2017;8:54978–92. https://doi.org/10.18632/oncotarget.18989.
Konda JD, Olivero M, Musiani D, Lamba S, Di Renzo MF. Heat-shock protein 27 (HSP27, HSPB1) is synthetic lethal to cells with oncogenic activation of MET, EGFR and BRAF. Mol Oncol. 2017;11:599–611. https://doi.org/10.1002/1878-0261.12042.
Zhao L, Li ZG, Ding YQ. Expression of HSP27 in colorectal carcinoma and its relationship with lymphatic metastasis. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:41–4.
Acknowledgements
This study was supported by a grant from the Natural Science Foundation of the Shanghai Science and Technology Commission (No. 16ZR1400800).
Author information
Authors and Affiliations
Contributions
MC conceived the study and designed the experiments. X-HJ and Y-GH provided the experimental materials. PL performed the experiments. L-QH performed the data analysis. X-HJ wrote the manuscript. All authors contributed to the interpretation and discussion of the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors. This study was approved by the research ethics committee of Changhai Hospital, Second Military Medical University and was performed in accordance with the Declaration of Helsinki. Informed written consent for the use of the tissue samples was obtained from all patients. The approval number of the Ethics Committee was 2017-148-01.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
**, XH., Hong, YG., Li, P. et al. Long noncoding RNA LINC00520 accelerates the progression of colorectal cancer by serving as a competing endogenous RNA of microRNA-577 to increase HSP27 expression. Human Cell 33, 683–694 (2020). https://doi.org/10.1007/s13577-020-00336-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00336-8