Abstract
Background
Current high lung cancer mortality rates are mainly due to the occurrence of metastases and therapeutic resistance. Therefore, simultaneous targeting of these processes may be a valid approach for the treatment of this type of cancer. Here, we assessed relationships between CXC chemokine receptor type 4 (CXCR4) and focal adhesion kinase (FAK) gene expression levels and expression levels of the drug resistance-related genes ABCB1 and ABCC1, and tested the potential of CXCR4 and FAK inhibitors to reverse doxorubicin (DOX) resistance and to decrease the invasive capacity of non-small cell lung carcinoma (NSCLC) cells.
Methods
qRT-PCR was used for gene expression analyses in primary lung tissue samples obtained from 30 NSCLC patients and the human NSCLC-derived cell lines NCI-H460, NCI-H460/R and COR-L23. MTT, flow cytometry, cell death and β-galactosidase activity assays were used to assess the in vitro impact of CXCR4 and FAK inhibitors on DOX sensitivity. In addition, invasion and gelatin degradation assays were used to assess the in vitro impact of the respective inhibitors on metastasis-related processes in combination with DOX treatment.
Results
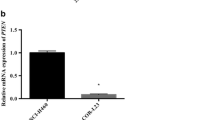
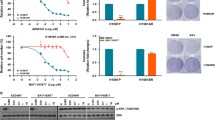
We found that ABCB1 over-expression was significantly associated with CXCR4 and FAK over-expression, whereas ABCC1 over-expression was associated with increased FAK expression. We also found that CXCR4 and FAK inhibitors strongly synergized with DOX in reducing cell viability, arresting the cell cycle in the S or G2/M phases and inducing senescence. Additionally, we found that DOX enhanced the anti-invasive potential of CXCR4 and FAK inhibitors by reducing gelatin degradation and invasion.
Conclusions
From our data we conclude that targeting of CXCR4 and FAK may overcome ABCB1 and ABCC1-dependent DOX resistance in NSCLC cells and that simultaneous treatment of these cells with DOX may potentiate the anti-invasive effects of CXCR4 and FAK inhibitors.







Similar content being viewed by others
References
C. Zeng, W. Fan, X. Zhang, RRM1 expression is associated with the outcome of gemcitabine-based treatment of non-small cell lung cancer patients--a short report. Cell. Oncol. 38, 319–325 (2015). doi:10.1007/s13402-015-0225-9
Z. Birsu Cincin, M. Unlu, B. Kiran, E. Sinem Bireller, Y. Baran, B. Cakmakoglu, Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell. Oncol. 38, 195–204 (2015). doi:10.1007/s13402-015-0222-z
B. Passlick, J. R. Izbicki, B. Kubuschok, W. Nathrath, O. Thetter, U. Pichlmeier, L. Schweiberer, G. Riethmuller, K. Pantel, Immunohistochemical assessment of individual tumor cells in lymph nodes of patients with non-small-cell lung cancer. J. Clin. Oncol. 12, 1827–1832 (1994)
S. A. Leon, B. Shapiro, D. M. Sklaroff, M. J. Yaros, Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 37, 646–650 (1977)
C. Holohan, S. Van Schaeybroeck, D. B. Longley, P. G. Johnston, Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013). doi:10.1038/nrc3599
Y. L. Chen, T. Y. Yang, K. C. Chen, C. L. Wu, S. L. Hsu, C. M. Hsueh, Hypoxia can impair doxorubicin resistance of non-small cell lung cancer cells by inhibiting MRP1 and P-gp expression and boosting the chemosensitizing effects of MRP1 and P-gp blockers. Cell. Oncol. 39, 411–433 (2016). doi:10.1007/s13402-016-0285-5
J. A. Burger, D. J. Stewart, O. Wald, A. Peled, Potential of CXCR4 antagonists for the treatment of metastatic lung cancer. Expert Rev. Anticancer Ther. 11, 621–630 (2011). doi:10.1586/era.11.11
F. Balkwill, The significance of cancer cell expression of the chemokine receptor CXCR4. Semin. Cancer Biol. 14, 171–179 (2004). doi:10.1016/j.semcancer.2003.10.003
K. Oonakahara, W. Matsuyama, I. Higashimoto, M. Kawabata, K. Arimura, M. Osame, Stromal-derived factor-1alpha/CXCL12-CXCR 4 axis is involved in the dissemination of NSCLC cells into pleural space. Am. J. Respir. Cell. Mol. Biol. 30, 671–677 (2004). doi:10.1165/rcmb.2003-0340OC
M. J. Jung, J. K. Rho, Y. M. Kim, J. E. Jung, Y. B. **, Y. G. Ko, J. S. Lee, S. J. Lee, J. C. Lee, M. J. Park, Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene 32, 209–221 (2013). doi:10.1038/onc.2012.37
B. C. Lee, T. H. Lee, S. Avraham, H. K. Avraham, Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol. Cancer Res. 2, 327–338 (2004)
N. K. Mukhopadhyay, G. J. Gordon, C. J. Chen, R. Bueno, D. J. Sugarbaker, M. T. Jaklitsch, Activation of focal adhesion kinase in human lung cancer cells involves multiple and potentially parallel signaling events. J. Cell. Mol. Med. 9, 387–397 (2005)
H. Lu, L. Wang, W. Gao, J. Meng, B. Dai, S. Wu, J. Minna, J. A. Roth, W. L. Hofstetter, S. G. Swisher, B. Fang, IGFBP2/FAK pathway is causally associated with dasatinib resistance in non-small cell lung cancer cells. Mol. Cancer Ther. 12, 2864–2873 (2013). doi:10.1158/1535-7163.MCT-13-0233
T. Andjelkovic, J. Bankovic, J. Stojsic, V. Milinkovic, A. Podolski-Renic, S. Ruzdijic, N. Tanic, Coalterations of p53 and PTEN tumor suppressor genes in non-small cell lung carcinoma patients. Transl. Res. 157, 19–28 (2011). doi:10.1016/j.trsl.2010.09.004
M. Pesic, J. Z. Markovic, D. Jankovic, S. Kanazir, I. D. Markovic, L. Rakic, S. Ruzdijic, Induced resistance in the human non small cell lung carcinoma (NCI-H460) cell line in vitro by anticancer drugs. J. Chemother. 18, 66–73 (2006). doi:10.1179/joc.2006.18.1.66
T. C. Chou, P. Talalay, Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22, 27–55 (1984)
X. Dai, Z. Mao, J. Huang, S. **e, H. Zhang, The CXCL12/CXCR4 autocrine loop increases the metastatic potential of non-small cell lung cancer in vitro. Oncol. Lett. 5, 277–282 (2013). doi:10.3892/ol. 2012.960
B. S. Ko, T. C. Chang, C. H. Chen, C. C. Liu, C. C. Kuo, C. Hsu, Y. C. Shen, T. L. Shen, V. M. Golubovskaya, C. C. Chang, S. K. Shyue, J. Y. Liou, Bortezomib suppresses focal adhesion kinase expression via interrupting nuclear factor-kappa B. Life Sci. 86, 199–206 (2010). doi:10.1016/j.lfs.2009.12.003
S. Bosch, S. Siavoshian, C. Jacquot, C. Tomasoni, G. Dabouis, Y. Elanbaloussi, T. Leneel, M. T. More, C. Roussakis, Correlation between multidrug resistance and the degree of differentiation of non-small-cell bronchopulmonary carcinoma (NSCLC) in vitro and in vivo. Anticancer Res. 17, 4595–4598 (1997)
T. Chadderton, C. Wilson, M. Bewick, S. Gluck, Evaluation of three rapid RNA extraction reagents: relevance for use in RT-PCR's and measurement of low level gene expression in clinical samples. Cell. Mol. Biol. 43, 1227–1234 (1997)
R. NicAmhlaoibh, M. Heenan, I. Cleary, S. Touhey, C. O'Loughlin, C. Daly, G. Nunez, K. J. Scanlon, M. Clynes, Altered expression of mRNAs for apoptosis-modulating proteins in a low level multidrug resistant variant of a human lung carcinoma cell line that also expresses mdr1 mRNA. Int. J. Cancer. 82, 368–376 (1999)
K. J. Livak, T. D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25, 402–408 (2001). doi:10.1006/meth.2001.1262
U. K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970)
T. Andjelkovic, M. Pesic, J. Bankovic, N. Tanic, I. D. Markovic, S. Ruzdijic, Synergistic effects of the purine analog sulfinosine and curcumin on the multidrug resistant human non-small cell lung carcinoma cell line (NCI-H460/R). Cancer Biol. Ther. 7, 1024–1032 (2008)
H. F. Ji, D. Pang, S. B. Fu, Y. **, L. Yao, J. P. Qi, J. Bai, Overexpression of focal adhesion kinase correlates with increased lymph node metastasis and poor prognosis in non-small-cell lung cancer. J. Cancer Res. Clin. Oncol. 139, 429–435 (2013). doi:10.1007/s00432-012-1342-8
S. Carelli, G. Zadra, V. Vaira, M. Falleni, L. Bottiglieri, M. Nosotti, A. M. Di Giulio, A. Gorio, S. Bosari, Up-regulation of focal adhesion kinase in non-small cell lung cancer. Lung Cancer 53, 263–271 (2006). doi:10.1016/j.lungcan.2006.06.001
E. Ota, Y. Abe, Y. Oshika, Y. Ozeki, M. Iwasaki, H. Inoue, H. Yamazaki, Y. Ueyama, K. Takagi, T. Ogata, et al., Expression of the multidrug resistance-associated protein (MRP) gene in non-small-cell lung cancer. Br. J. Cancer 72, 550–554 (1995)
K. Nooter, F. T. Bosman, H. Burger, K. E. van Wingerden, M. J. Flens, R. J. Scheper, R. G. Oostrum, A. W. Boersma, A. van der Gaast, G. Stoter, Expression of the multidrug resistance-associated protein (MRP) gene in primary non-small-cell lung cancer. Ann. Oncol. 7, 75–81 (1996)
W. Berger, U. Setinek, P. Hollaus, T. Zidek, E. Steiner, L. Elbling, H. Cantonati, J. Attems, A. Gsur, M. Micksche, Multidrug resistance markers P-glycoprotein, multidrug resistance protein 1, and lung resistance protein in non-small cell lung cancer: prognostic implications. J. Cancer Res. Clin. Oncol. 131, 355–363 (2005). doi:10.1007/s00432-004-0653-9
L. Su, J. Zhang, H. Xu, Y. Wang, Y. Chu, R. Liu, S. **ong, Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin. Cancer Res. 11, 8273–8280 (2005). doi:10.1158/1078-0432.CCR-05-0537
A. Owen, B. Chandler, P. G. Bray, S. A. Ward, C. A. Hart, D. J. Back, S. H. Khoo, Functional correlation of P-glycoprotein expression and genotype with expression of the human immunodeficiency virus type 1 coreceptor CXCR4. J. Virol. 78, 12022–12029 (2004). doi:10.1128/jvi.78.21.12022-12029.2004
J. H. Lee, A. Nan, Combination drug delivery approaches in metastatic breast cancer. J Drug Deliv 2012, 915375 (2012). doi:10.1155/2012/915375
A. G. Pallis, L. Serfass, R. Dziadziusko, J. P. van Meerbeeck, D. Fennell, D. Lacombe, J. Welch, C. Gridelli, Targeted therapies in the treatment of advanced/metastatic NSCLC. Eur. J. Cancer 45, 2473–2487 (2009). doi:10.1016/j.ejca.2009.06.005
A. F. Dessein, L. Stechly, N. Jonckheere, P. Dumont, D. Monte, E. Leteurtre, S. Truant, F. R. Pruvot, M. Figeac, M. Hebbar, C. H. Lecellier, T. Lesuffleur, R. Dessein, G. Grard, M. J. Dejonghe, Y. de Launoit, Y. Furuichi, G. Prevost, N. Porchet, C. Gespach, G. Huet, Autocrine induction of invasive and metastatic phenotypes by the MIF-CXCR4 axis in drug-resistant human colon cancer cells. Cancer Res. 70, 4644–4654 (2010). doi:10.1158/0008-5472.CAN-09-3828
L. Mei, Y. Liu, Q. Zhang, H. Gao, Z. Zhang, Q. He, Enhanced antitumor and anti-metastasis efficiency via combined treatment with CXCR4 antagonist and liposomal doxorubicin. J. Control Release 196, 324–331 (2014). doi:10.1016/j.jconrel.2014.10.017
C. Chittasupho, K. Lirdprapamongkol, P. Kewsuwan, N. Sarisuta, Targeted delivery of doxorubicin to A549 lung cancer cells by CXCR4 antagonist conjugated PLGA nanoparticles. Eur. J. Pharm. Biopharm. 88, 529–538 (2014). doi:10.1016/j.ejpb.2014.06.020
Y. Wang, H. Miao, W. Li, J. Yao, Y. Sun, Z. Li, L. Zhao, Q. Guo, CXCL12/CXCR4 axis confers adriamycin resistance to human chronic myelogenous leukemia and oroxylin a improves the sensitivity of K562/ADM cells. Biochem. Pharmacol. 90, 212–225 (2014). doi:10.1016/j.bcp.2014.05.007
E. V. Kurenova, D. L. Hunt, D. He, A. T. Magis, D. A. Ostrov, W. G. Cance, Small molecule chloropyramine hydrochloride (C4) targets the binding site of focal adhesion kinase and vascular endothelial growth factor receptor 3 and suppresses breast cancer growth in vivo. J. Med. Chem. 52, 4716–4724 (2009). doi:10.1021/jm900159g
J. E. Stewart, X. Ma, M. Megison, H. Nabers, W. G. Cance, E. V. Kurenova, E. A. Beierle, Inhibition of FAK and VEGFR-3 binding decreases tumorigenicity in neuroblastoma. Mol. Carcinog. 54, 9–23 (2015). doi:10.1002/mc.22070
V. M. Golubovskaya, B. Sumbler, B. Ho, M. Yemma, W. G. Cance, MiR-138 and MiR-135 directly target focal adhesion kinase, inhibit cell invasion, and increase sensitivity to chemotherapy in cancer cells. Anticancer Agents Med. Chem. 14, 18–28 (2014)
A. Datta, N. Bhasin, H. Kim, M. Ranjan, B. Rider, Z. Y. Abd Elmageed, D. Mondal, K. C. Agrawal, A. B. Abdel-Mageed, Selective targeting of FAK-Pyk2 axis by alpha-naphthoflavone abrogates doxorubicin resistance in breast cancer cells. Cancer Lett. 362, 25–35 (2015). doi:10.1016/j.canlet.2015.03.009
A. K. Azab, J. M. Runnels, C. Pitsillides, A. S. Moreau, F. Azab, X. Leleu, X. Jia, R. Wright, B. Ospina, A. L. Carlson, C. Alt, N. Burwick, A. M. Roccaro, H. T. Ngo, M. Farag, M. R. Melhem, A. Sacco, N. C. Munshi, T. Hideshima, B. J. Rollins, K. C. Anderson, A. L. Kung, C. P. Lin, I. M. Ghobrial, CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 113, 4341–4351 (2009). doi:10.1182/blood-2008-10-186668
B. Tavora, L. E. Reynolds, S. Batista, F. Demircioglu, I. Fernandez, T. Lechertier, D. M. Lees, P. P. Wong, A. Alexopoulou, G. Elia, A. Clear, A. Ledoux, J. Hunter, N. Perkins, J. G. Gribben, K. M. Hodivala-Dilke, Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature 514, 112–116 (2014). doi:10.1038/nature13541
N. Song, A. J. Kim, H. J. Kim, H. J. Jee, M. Kim, Y. H. Yoo, J. Yun, Melatonin suppresses doxorubicin-induced premature senescence of A549 lung cancer cells by ameliorating mitochondrial dysfunction. J. Pineal Res. 53, 335–343 (2012). doi:10.1111/j.1600-079X.2012.01003.x
Y. Pylayeva, K. M. Gillen, W. Gerald, H. E. Beggs, L. F. Reichardt, F. G. Giancotti, Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J. Clin. Invest. 119, 252–266 (2009). doi:10.1172/jci37160
G. K. Dy, The role of focal adhesion kinase in lung cancer. Anticancer Agents Med. Chem. 13, 581–583 (2013)
S. **e, W. Zeng, G. Fan, J. Huang, G. Kang, Q. Geng, B. Cheng, W. Wang, P. Dong, Effect of CXCL12/CXCR4 on increasing the metastatic potential of non-small cell lung cancer is inhibited through the downregulation of CXCR4 chemokine receptor expression. Oncol. Lett. 7, 941–947 (2014). doi:10.3892/ol.2014.1837
A. K. Singla, C. M. Downey, G. D. Bebb, F. R. Jirik, Characterization of a murine model of metastatic human non-small cell lung cancer and effect of CXCR4 inhibition on the growth of metastases. Oncoscience 2, 263–271 (2015)
Acknowledgments
This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant No III41031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The patient samples were collected and used in the study after obtaining informed consents and approval from the Ethics Committee, in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dragoj, M., Milosevic, Z., Bankovic, J. et al. Targeting CXCR4 and FAK reverses doxorubicin resistance and suppresses invasion in non-small cell lung carcinoma. Cell Oncol. 40, 47–62 (2017). https://doi.org/10.1007/s13402-016-0304-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-016-0304-6