Abstract
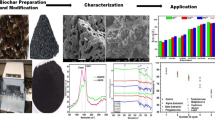
In the present study, microporous biochar was prepared from the waste plant fruit shell of Pterospermum acerifolium. To improve porosity and nitrogen content in biochar, the powder form of the fruit shell was pre-treated by HNO3 before the biochar synthesis. Furthermore, to enhance the adsorption capacity of biochar for cationic methylene blue (MB) dye, the surface of biochar was modified by sodium dodecyl sulfate (SDS) surfactant to increase the negative charge density on the biochar surface. Before the performance of the adsorption experiments, the nitric acid-treated Pterospermum acerifolium fruit waste biochar (NAT-PABC) and SDS-modified nitric acid-treated Pterospermum acerifolium fruit waste biochar (SDS-NAT-PABC) were characterized by various sophisticated instruments such as FTIR, XRD, FE-SEM, EDX, elemental map**, BET, XPS, and point of zero charge. An in-depth study of the functional groups and their interactions in NAT-PABC and SDS-NAT-PABC were examined with the help of the XPS technique and identified the functional groups responsible for the adsorption of MB dye. Batch adsorption experiments were carried out to determine the optimal adsorption conditions. The maximum removal percentage of MB dye was achieved at pH 10 and 9 for NAT-PABC and SDS-NAT-PABC, respectively, within 240 min. The isotherm, kinetic, and mass transfer modeling were also examined and fitted with the experimental data. According to correlation coefficient (R2), nonlinear forms of the pseudo-second-order kinetic and Langmuir isotherm models were best suited for both NAT-PABC and SDS-NAT-PABC. The present work also reported a possible reaction mechanism that describes the adsorption phenomenon. The Gibbs energy and enthalpy for the MB adsorption process by NAT-PABC and SDS-NAT-PABC was found to be negative and positive, respectively, suggesting that the process was spontaneous and endothermic nature; therefore, the reaction was highly attainable at higher temperatures.















Similar content being viewed by others
References
Iwuozor KO, Ighalo JO, Ogunfowora LA, Adeniyi AG, Igwegbe CA (2021) An empirical literature analysis of adsorbent performance for methylene blue uptake from aqueous media. J Environ Chem Eng 9:105658
Prajapati AK, Mondal MK (2019) Hazardous As (III) removal using nanoporous activated carbon of waste garlic stem as adsorbent: kinetic and mass transfer mechanisms. Korean J Chem Eng 36(11):1900–1914
Kumar V, Sharma A, Kumar R, Bhardwaj R, Kumar Thukral A, Rodrigo-Comino J (2020) Assessment of heavy-metal pollution in three different Indian water bodies by combination of multivariate analysis and water pollution indices. Hum Ecol Risk Assess: An Int J 26(1):1–16
Prajapati AK, Mondal MK (2020) Comprehensive kinetic and mass transfer modeling for methylene blue dye adsorption onto CuO nanoparticles loaded on nanoporous activated carbon prepared from waste coconut shell. J Mol Liquids 307:112949
Prajapati AK, Mondal MK (2021) Development of CTAB modified ternary phase α-Fe2O3-Mn2O3-Mn3O4 nanocomposite as innovative super-adsorbent for Congo red dye adsorption. J Environ Chem Eng 9(1):104827
Padhi B (2012) Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int J Environ Sci 3(3):940–955
Ighalo JO, Iwuozor KO, Igwegbe CA, Adeniyi AG (2021) Verification of pore size effect on aqueous-phase adsorption kinetics: a case study of methylene blue. Colloids Surf A: Physicochem Eng Aspects 626:127119
Goluboff N, Wheaton R (1961) Methylene blue induced cyanosis and acutehemolytic anemia complicating the treatment of methemoglobinemia. J Pediatr 58(1):86–89
Jawad AH, Abdulhameed AS, Mastuli MS (2020) Acid-factionalized biomass material for methylene blue dye removal: a comprehensive adsorption and mechanism study. J Taibah Univ Sci 14(1):305–313
Shah NS, Khan JA, Sayed M, Khan ZU, Iqbal J, Arshad S, Junaid M, Khan HM (2020) Synergistic effects of H2O2 and S2O82− in the gamma radiation induced degradation of congo-red dye: Kinetics and toxicities evaluation. Sep Purif Technol 233:115966
Sharma G, Kumar A, Sharma S, Naushad M, Dhiman P, Vo DV, Stadler FJ (2020) Fe3O4/ZnO/Si3N4 nanocomposite based photocatalyst for the degradation of dyes from aqueous solution. Mater Lett 278:128359
Hachemaoui M, Boukoussa B, Mokhtar A, Mekki A, Beldjilali M, Benaissa M, Zaoui F, Hakiki A, Chaibi W, Sassi M (2020) Dyes adsorption, antifungal and antibacterial properties of metal loaded mesoporous silica: effect of metal and calcination treatment. Mater Chem Phys 256:123704
Prajapati AK, Mondal MK (2021) Novel green strategy for CuO–ZnO–C nanocomposites fabrication using marigold (Tagetes spp.) flower petals extract with and without CTAB treatment for adsorption of Cr (VI) and Congo red dye. J Environ Manag 290:112615
Ibrahim WMHW, Amini MHM, Sulaiman NS, Kadir WRWA (2021) Evaluation of alkaline-based activated carbon from Leucaena Leucocephala produced at different activation temperatures for cadmium adsorption. Appl Water Sci 11(1):1–13
Panahi HKS, Dehhaghi M, Ok YS, Nizami A-S, Khoshnevisan B, Mussatto SI, Aghbashlo M, Tabatabaei M, Lam SS (2020) A comprehensive review of engineered biochar: production, characteristics, and environmental applications. J Clean Prod 270:122462
Pathania D, Srivastava A (2021) Advances in nanoparticles tailored lignocellulosic biochars for removal of heavy metals with special reference to cadmium (II) and chromium (VI). Environ Sustain 4(2):201–214
Wan X, Li C, Parikh SJ (2020) Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ Pollut 261:114157
Amin MT, Alazba AA, Shafiq M (2021) Successful application of Eucalyptus camdulensis biochar in the batch adsorption of crystal violet and methylene blue dyes from aqueous solution. Sustainability 13(7):3600
Sun Y, Iris K, Tsang DC, Fan J, Clark JH, Luo G, Zhang S, Khan E, Graham NJ (2020) Tailored design of graphitic biochar for high-efficiency and chemical-free microwave-assisted removal of refractory organic contaminants. Chem Eng J 398:125505
Wang Z, Ren D, Zhao Y, Huang C, Zhang S, Zhang X, Kang C, Deng Z, Guo H (2021) Remediation and improvement of 2, 4-dichlorophenol contaminated soil by biochar-immobilized laccase. Environ Technol 42(11):1679–1692
Panwar, N. Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: a review, Biomass Convers Biorefin (2020) 1–23.
Tran HN, Tomul F, Ha NTH, Nguyen DT, Lima EC, Le GT, Chang C-T, Masindi V, Woo SH (2020) Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J Hazard Mater 394:122255
Shirani Z, Song H, Bhatnagar A (2020) Efficient removal of diclofenac and cephalexin from aqueous solution using Anthriscus sylvestris-derived activated biochar. Sci Total Environ 745:140789
Mansouri F, Chouchene K, Roche N, Ksibi M (2021) Removal of pharmaceuticals from water by adsorption and advanced oxidation processes: state of the art and trends. Appl Sci 11(14):6659
Park J-H, Wang JJ, Meng Y, Wei Z, DeLaune RD, Seo D-C (2019) Adsorption/desorption behavior of cationic and anionic dyes by biochars prepared at normal and high pyrolysis temperatures. Colloids Surf, A 572:274–282
Li G, Zhu W, Zhang C, Zhang S, Liu L, Zhu L, Zhao W (2016) Effect of a magnetic field on the adsorptive removal of methylene blue onto wheat straw biochar. Biores Technol 206:16–22
Sun L, Chen D, Wan S, Yu Z (2015) Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Biores Technol 198:300–308
Singh, S. Prajapati, A.K. Chakraborty, J.P. Mondal, M.K. Adsorption potential of biochar obtained from pyrolysis of raw and torrefied Acacia nilotica towards removal of methylene blue dye from synthetic wastewater, Biomass Convers Biorefin (2021) 1–22.
Nanda S, Mohanty P, Pant KK, Naik S, Kozinski JA, Dalai AK (2013) Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenergy Res 6(2):663–677
Usman AR, Abduljabbar A, Vithanage M, Ok YS, Ahmad M, Ahmad M, Elfaki J, Abdulazeem SS, Al-Wabel MI (2015) Biochar production from date palm waste: charring temperature induced changes in composition and surface chemistry. J Anal Appl Pyrol 115:392–400
Kumar P, Prajapati AK, Dixit S, Yadav VL (2020) Adsorption of fluoride from aqueous solution using biochar prepared from waste peanut hull. Mater Res Express 6(12):125553
Prajapati AK, Das S, Mondal MK (2020) Exhaustive studies on toxic Cr (VI) removal mechanism from aqueous solution using activated carbon of Aloe vera waste leaves. J Mol Liquids 307:112956
Marrakchi F, Auta M, Khanday W, Hameed B (2017) High-surface-area and nitrogen-rich mesoporous carbon material from fishery waste for effective adsorption of methylene blue. Powder Technol 321:428–434
Güzel F, Sayğılı H, Sayğılı GA, Koyuncu F, Yılmaz C (2017) Optimal oxidation with nitric acid of biochar derived from pyrolysis of weeds and its application in removal of hazardous dye methylene blue from aqueous solution. J Clean Prod 144:260–265
Que W, Jiang L, Wang C, Liu Y, Zeng Z, Wang X, Ning Q, Liu S, Zhang P, Liu S (2018) Influence of sodium dodecyl sulfate coating on adsorption of methylene blue by biochar from aqueous solution. J Environ Sci 70:166–174
Fernando JC, Peiris C, Navarathna CM, Gunatilake SR, Welikala U, Wanasinghe ST, Madduri SB, Jayasinghe S, Mlsna TE, Ferez F (2021) Nitric acid surface pre-modification of novel Lasia spinosa biochar for enhanced methylene blue remediation. Groundw Sustain Dev 14:100603
Sahu S, Pahi S, Tripathy S, Singh SK, Behera A, Sahu UK, Patel RK (2020) Adsorption of methylene blue on chemically modified lychee seed biochar: dynamic, equilibrium, and thermodynamic study. J Mol Liquids 315:113743
Dos Santos KJL, Dos Santos GEdS, de Sá ÍMGL, Ide AH, da Silva Duarte JL, de Carvalho SHV, Soletti JI, Meili L (2019) Wodyetia bifurcata biochar for methylene blue removal from aqueous matrix. Bioresour Technol 293:122093
Heviraa L, Zilfac Rahmayeni, Ighalo JO, Aziz H, Zein R (2021) Terminalia catappa shell as low-cost biosorbent for the removal of methylene blue from aqueous solutions. J Ind Eng Chem 97:188–199
Albadarin AB, Collins MN, Naushad M, Shirazian S, Walker G, Mangwandi C (2017) Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem Eng J 307:264–272
Alomar TS, AlMasoud N, Sharma G, ALOthman ZA, Naushad M (2021) Incorporation of trimetallic nanoparticles to the SiO2 matrix for the removal of methylene blue dye from aqueous medium. J Mol Liquids 336:116274
Prajapati, A.K. Mondal, M.K. Green synthesis of Fe3O4-onion peel biochar nanocomposites for adsorption of Cr(VI), methylene blue and congo red dye from aqueous solutions, J Mol Liquids (2021) 118161.
Fan S, Wang Y, Wang Z, Tang J, Tang J, Li X (2017) Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: adsorption kinetics, equilibrium, thermodynamics and mechanism. J Environ Chem Eng 5(1):601–611
Acknowledgements
The authors are thankful to the Department of Chemical Engineering, Indian Institute of Technology (IIT ISM) Dhanbad, India, for their support. The authors are also grateful to Indian Institute of Technology, Varanasi, India, for the characterization support. The author A. Oraon wants to acknowledge the whole team who supported him during this research study beside many difficulties faced in our mother institution BIT Sindri, Dhanbad, India.
Author information
Authors and Affiliations
Contributions
Ajay Oraon: data curation, investigation, formal analysis, methodology, writing—original draft; Anuj Kumar Prajapati: conceptualization, investigation, validation and software analysis; Mahendra Ram: conceptualization, writing—review and editing; Vinod Kumar Saxena: investigation, validation; Suman Dutta: supervision, validation, writing—review; Amit Kumar Gupta: investigation, validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oraon, A., Prajapati, A.K., Ram, M. et al. Synthesis, characterization, and application of microporous biochar prepared from Pterospermum acerifolium plant fruit shell waste for methylene blue dye adsorption: the role of surface modification by SDS surfactant. Biomass Conv. Bioref. 14, 931–953 (2024). https://doi.org/10.1007/s13399-022-02320-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02320-8