Abstract
The biochar produced from agricultural crop residues through thermochemical processes helps in crop waste management. Biochar has paid more attention due to its distinctive features such as high organic carbon content, stable structure, large surface area, and cation exchange capacity. The biochar obtained from crop residues can be readily converted into activated biochar. This review paper systematically summarised the preparation of activated biochar, characterisation, and analytical techniques of activated biochar based on more than 150 literatures published in the last 10 years. The physicochemical properties of activated biochar varies according to the type of feedstock, pyrolysis condition, and mode of activation. The selection of the activation method mainly depends on its further end environmental application. Physical activation or steam purging at high temperature creates pores inside biochar. Gas purging increases the surface area and pore volume, although steam activation is not much suitable to improve the BC surface functionality as compared to chemical and impregnation activation. Sulphuric and oxalic acid–modified biochar was found to be most suitable for the soil amendment. Alkaline activation enhances the surface area and oxygen-containing functional group in activated biochar. Metal oxide–modified biochar had better surface functionalities than did physical- and chemical-activated biochar and better sorption of organic and inorganic contaminants from potable water and waste water. In summary, activated biochar has a wide environmental prospect in remediation.

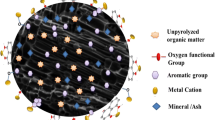
Graphical abstract




Similar content being viewed by others
References
Ahmed MB, Zhou JL, Ngo HH, Guo W (2015) Adsorptive removal of antibiotics from water and wastewater: progress and challenges. Sci Total Environ 532:112–126
Mohan D, Sarswat A, Ok YS, Pittman CU Jr (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent–a critical review. Bioresour Technol 160:191–202
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Novak JM, Lima I, **ng B et al (2009) Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann Environ Sci 3:195–206
Vithanage M, Rajapaksha AU, Ahmad M, Uchimiya M, Dou X, Alessi DS, Ok YS (2015) Mechanisms of antimony adsorption onto soybean stover-derived biochar in aqueous solutions. J Environ Manag 151:443–449
Ma Y, Liu WJ, Zhang N, Li YS, Jiang H, Sheng GP (2014) Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour Technol 169:403–408
Wang MC, Sheng GD, Qiu YP (2015) A novel manganese-oxide/biochar composite for efficient removal of lead (II) from aqueous solutions. Int J Environ Sci Technol 12:1719–1726
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Shen W, Li Z, Liu Y (2008) Surface chemical functional groups modification of porous carbon. Recent Patents on Chem Eng 1:27–40
Cha JS, Park SH, Jung SC, Ryu C, Jeon JK, Shin MC, Park YK (2016) Production and utilization of biochar: a review. J Ind Eng Chem 40:1–15
Collard FX, Blin J (2014) A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sust Energ Rev 38:594–608
Downie AE, Van Zwieten L, Smernik RJ et al (2011) Terra Preta Australis: reassessing the carbon storage capacity of temperate soils. Agric Ecosyst Environ 140:137–147
Huggins TM, Haeger A, Biffinger JC, Ren ZJ (2016) Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Res 94:225–232
Tan G, Sun W, Xu Y, Wang H, Xu N (2016) Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour Technol 211:727–735
Nair V, Vinu R (2016) Peroxide-assisted microwave activation of pyrolysis char for adsorption of dyes from wastewater. Bioresour Technol 216:511–519
Yao Y, Gao B, Chen JJ, Yang L (2013) Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slowrelease fertilizer. Environ Sci Technol 47:8700–8708
Delgado LF, Charles P, Glucina K et al (2012) The removal of endocrine disrupting compounds, pharmaceutically activated compounds and cyanobacterial toxins during drinking water preparation using activated carbon—a review. Sci Total Environ 435:509–525
Sevilla M, Mokaya R (2014) Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ Sci 7:1250–1280
Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrolysis 89:143–151
Lee HW, Kim YM, Kim S et al (2018) Review of the use of activated biochar for energy and environmental applications. Carbon Lett (Carbon Lett) 26:1–10
Ahmad M, Lee SS, Lim JE, Lee SE, Cho JS, Moon DH, Hashimoto Y, Ok YS (2014) Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 95:433–441
Wang J, Zhuan R, Chu L (2019) The occurrence, distribution and degradation of antibiotics by ionizing radiation: an overview. Sci Total Environ 646:1385–1397
Zhang K, Sun P, Zhang Y (2019) Decontamination of Cr (VI) facilitated formation of persistent free radicals on rice husk derived biochar. Front Environ Sci Eng 13:22
Kalinke C, Oliveira PR, Oliveira GA, Mangrich AS, Marcolino-Junior LH, Bergamini MF (2017) Activated biochar: preparation, characterization and electroanalytical application in an alternative strategy of nickel determination. Anal Chim Acta 983:103–111
Adhikari S, Sajib S (2020) Effect of pyrolysis methods on physical properties of activated biochar and its application as cathode electrode for lithium-sulfur battery. Trans ASABE 63:485–493
**ao Y, Zhang A, Liu S, Zhao J, Fang S, Jia D, Li F (2012) Free-standing and porous hierarchical nanoarchitectures constructed with cobalt cobaltite nanowalls for supercapacitors with high specific capacitances. J Power Sources 219:140–146
Ahn SY, Eom SY, Rhie YH, Sung YM, Moon CE, Choi GM, Kim DJ (2013) Utilization of wood biomass char in a direct carbon fuel cell (DCFC) system. Appl Energy 105:207–216
Alonso DM, Wettstein SG, Dumesic JA (2012) Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem Soc Rev 41:8075–8098
Yahya MA, Al-Qodah Z, Ngah CZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sust Energ Rev 46:218–235
Roman S, Nabais JV, Ledesma B et al (2013) Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater 165:127–133
Mui EL, Cheung WH, Valix M et al (2010) Activated carbons from bamboo scaffolding using acid activation. Sep Purif Technol 74:213–218
Petkovic LM, Ginosar DM, Rollins HW et al (2009) Activated carbon catalysts for the production of hydrogen via the sulfur–iodine thermochemical water splitting cycle. Int J Hydrog Energy 34:4057–4064
Sha Y, Lou J, Bai S, Wu D, Liu B, Ling Y (2015) Facile preparation of nitrogen-doped porous carbon from waste tobacco by a simple pre-treatment process and their application in electrochemical capacitor and CO2 capture. Mater Res Bull 64:327–332
Htwe WM, Kyawt YY, Thaikua S, Imai Y, Mizumachi S, Kawamoto Y (2016) Effects of liming on dry biomass, lead concentration and accumulated amounts in roots and shoots of three tropical pasture grasses from lead contaminated acidic soils. Grassl Sci 62:257–261
Gautam RK, Mudhoo A, Lofrano G, Chattopadhyaya MC (2014) Biomass-derived biosorbents for metal ions sequestration: adsorbent modification and activation methods and adsorbent regeneration. J Environ Chem Eng 2:239–259
Acemioğlu B (2019) Removal of a reactive dye using NaOH-activated biochar prepared from peanut shell by pyrolysis process. Int J Coal Prep Util 1–23. https://doi.org/10.1080/19392699.2019.1644326
Mohammed J, Nasri NS, Zaini MA et al (2015) Adsorption of benzene and toluene onto KOH activated coconut shell based carbon treated with NH3. Int Biodeterior Biodegradation 102:245–255
Yang W, Liu Y, Wang Q, Pan J (2017) Removal of elemental mercury from flue gas using wheat straw chars modified by Mn-Ce mixed oxides with ultrasonic-assisted impregnation. Chem Eng J 326:169–181
Hamza UD, Nasri NS, Amin NS et al (2016) Characteristics of oil palm shell biochar and activated carbon prepared at different carbonization times. Desalin Water Treat 57:7999–8006
Adinaveen T, Kennedy LJ, Vijaya JJ et al (2015) Surface and porous characterization of activated carbon prepared from pyrolysis of biomass (rice straw) by two-stage procedure and its applications in supercapacitor electrodes. J Mater Cycles Waste Manag 17:736–747
Köseoğlu E, Akmil-Başar C (2015) Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv Powder Technol 26:811–818
Ozdemir I, Şahin M, Orhan R, Erdem M (2014) Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process Technol 125:200–206
Tiryaki B, Yagmur E, Banford A, Aktas Z (2014) Comparison of activated carbon produced from natural biomass and equivalent chemical compositions. J Anal Appl Pyrolysis 105:276–283
Uysal T, Duman G, Onal Y, Yasa I, Yanik J (2014) Production of activated carbon and fungicidal oil from peach stone by two-stage process. J Anal Appl Pyrolysis 108:47–55
Largitte L, Brudey T, Tant T, Dumesnil PC, Lodewyckx P (2016) Comparison of the adsorption of lead by activated carbons from three lignocellulosic precursors. Microporous Mesoporous Mater 219:265–275
Hosseini S, Soltani SM, Jahangirian H et al (2015) Fabrication and characterization porous carbon rod-shaped from almond natural fibers for environmental applications. J Environ Chem Eng 3:2273–2280
Erabee IK, Ahsan A, Daud NN et al (2017) Manufacture of low-cost activated carbon using sago palm bark and date pits by physiochemical activation. Bioresources 12:1916–1923
Pallarés J, González-Cencerrado A, Arauzo I (2018) Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 115:64–73
Choi GG, Oh SJ, Lee SJ, Kim JS (2015) Production of bio-based phenolic resin and activated carbon from bio-oil and biochar derived from fast pyrolysis of palm kernel shells. Bioresour Technol 178:99–107
Sayğılı H, Güzel F (2016) High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J Clean Prod 113:995–1004
Suhas PJM, Carrott MML, Carrott R (2007) Lignin – from natural adsorbent to activated carbon: a review. Bioresour Technol 98:2301–2312
Zhao Y, Fang F, **ao HM, Feng QP, **ong LY, Fu SY (2015) Preparation of pore-size controllable activated carbon fibers from bamboo fibers with superior performance for xenon storage. Chem Eng J 270:528–534
Foo KY, Hameed BH (2012) Coconut husk derived activated carbon via microwave induced activation: effects of activation agents, preparation parameters and adsorption performance. Chem Eng J 184:57–65
González PG, Pliego-Cuervo YB (2013) Physicochemical and microtextural characterization of activated carbons produced from water steam activation of three bamboo species. J Anal Appl Pyrolysis 99:32–39
González-García P, Centeno TA, Urones-Garrote E, Ávila-Brande D, Otero-Díaz LC (2013) Microstructure and surface properties of lignocellulosic-based activated carbons. Appl Surf Sci 265:731–737
Abioye AM, Ani FN (2015) Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: a review. Renew Sust Energ Rev 52:1282–1293
Lua AC, Lau FY, Guo J (2006) Influence of pyrolysis conditions on pore development of oil-palm-shell activated carbons. J Anal Appl Pyrolysis 76:96–102
Ioannidou O, Zabaniotou A (2007) Agricultural residues as precursors for activated carbon production—a review. Renew Sust Energ Rev 11:1966–2005
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190:432–441
Park JH, Ok YS, Kim SH, Cho JS, Heo JS, Delaune RD, Seo DC (2015) Evaluation of phosphorus adsorption capacity of sesame straw biochar on aqueous solution: influence of activation methods and pyrolysis temperatures. Environ Geochem Health 37:969–983
Vithanage M, Rajapaksha AU, Zhang M, Thiele-Bruhn S, Lee SS, Ok YS, Thiele-Bruhn S, Lee SS, Ok YS (2015) Acid-activated biochar increased sulfamethazine retention in soils. Environ Sci Pollut Res 22:2175–2186
Zhao B, O'Connor D, Zhang J, Peng T, Shen Z, Tsang DCW, Hou D (2018) Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J Clean Prod 174:977–987
Al-Wabel MI, Al-Omran A, El-Naggar AH et al (2013) Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour Technol 131:374–379
González PG (2018) Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew Sust Energ Rev 82:1393–1414
Ali I (2010) The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater. Sep Purif Rev 39:95–171
Chen Y, Zhu Y, Wang Z, Li Y, Wang L, Ding L, Gao X, Ma Y, Guo Y (2011) Application studies of activated carbon derived from rice husks produced by chemical-thermal process—a review. Adv Colloid Interf Sci 163:39–52
Puziy AM, Poddubnaya OI, Martınez-Alonso A et al (2002) Characterization of synthetic carbons activated with phosphoric acid. Appl Surf Sci 200:196–202
Hadjittofi L, Prodromou M, Pashalidis I (2014) Activated biochar derived from cactus fibres–preparation, characterization and application on Cu (II) removal from aqueous solutions. Bioresour Technol 159:460–464
Giraldo L, Moreno-Pirajan JC (2012) Synthesis of activated carbon mesoporous from coffee waste and its application in adsorption zinc and mercury ions from aqueous solution. J Chemother 9:938–948
Dutta S, Kim J, Ide Y, Ho Kim J, Hossain MSA, Bando Y, Yamauchi Y, Wu KCW (2017) 3D network of cellulose-based energy storage devices and related emerging applications. Mater Horiz 4:522–545. https://doi.org/10.1039/C6MH00500D
Lukatskaya MR, Dunn B, Gogotsi Y (2016) Multidimensional materials and device architectures for future hybrid energy storage. Nat Commun 7:12647. https://doi.org/10.1038/ncomms12647
Xu G, Nie P, Dou H et al (2017) Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater Today 20:191–209
Bardestani R, Kaliaguine S (2018) Steam activation and mild air oxidation of vacuum pyrolysis biochar. Biomass Bioenergy 108:101–112
Vijayaraghavan K (2019) Recent advancements in biochar preparation, feedstocks, modification, characterization and future applications. Environ Technol Rev 8:47–64
Rajapaksha AU, Vithanage M, Ahmad M, Seo DC, Cho JS, Lee SE, Lee SS, Ok YS (2015) Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J Hazard Mater 290:43–50
Chakraborty P, Banerjee S, Kumar S, Sadhukhan S, Halder G (2018) Elucidation of ibuprofen uptake capability of raw and steam activated biochar of Aegle marmelos shell: isotherm, kinetics, thermodynamics and cost estimation. Process Saf Environ Prot 118:10–23
Sewu DD, Jung H, Kim SS, Lee DS, Woo SH (2019) Decolorization of cationic and anionic dye-laden wastewater by steam-activated biochar produced at an industrial-scale from spent mushroom substrate. Bioresour Technol 277:77–86
**ao F, Pignatello JJ (2015) Interactions of triazine herbicides with biochar: steric enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J Hazard Mater 90:43–50
Abu Bakar MS (2019) Comparison of chemical properties and adsorption capabilities of different pyrolysis temperature biochar and steam activated biochar derived from empty fruit bunch (EFB).
Olivares-Marín M, Fernández-González C, Macías-García A, Gómez-Serrano V (2012) Preparation of activated carbon from cherry stones by physical activation in air. Influence of the chemical carbonisation with H2SO4. J Anal Appl Pyrolysis 94:131–137
Ould-Idriss A, Stitou M, Cuerda-Correa EM, Fernández-González C, Macías-García A, Alexandre-Franco MF, Gómez-Serrano V (2011) Preparation of activated carbons from olive-tree wood revisited. II Physical activation with air. Fuel Process Technol 92:266–270
Kwiatkowski M, Broniek E (2017 Sep 20) An analysis of the porous structure of activated carbons obtained from hazelnut shells by various physical and chemical methods of activation. Colloids Surf A Physicochem Eng Asp 529:443–453
Shao J, Zhang J, Zhang X, Feng Y, Zhang H, Zhang S, Chen H (2018) Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation. Fuel 224:138–146
** Y, Tao S, Yingquan C, Han** C (2013) Influence of NH 3/CO 2 modification on the characteristic of biochar and the CO 2 capture. BioEnergy Res 6:1147–1153
Zhang X, Zhang S, Yang H, Feng Y, Chen Y, Wang X, Chen H (2014) Nitrogen enriched biochar modified by high temperature CO2–ammonia treatment: characterization and adsorption of CO2. Chem Eng J 257:20–27
Qian K, Kumar A, Zhang H, Bellmer D, Huhnke R (2015) Recent advances in utilization of biochar. Renew Sust Energ Rev 42:1055–1064
Aghababaei A, Ncibi MC, Sillanpää M (2017) Optimized removal of oxytetracycline and cadmium from contaminated waters using chemically-activated and pyrolyzed biochars from forest and wood-processing residues. Bioresour Technol 239:28–36
Issa AA, Al-Degs YS, El-Sheikh AH et al (2017) Application of partial least squares-kernel calibration in competitive adsorption studies using an effective chemically activated biochar. CLEAN–Soil Air Water 45:1600333
Hsu D, Lu C, Pang T, Wang Y, Wang G (2019) Adsorption of ammonium nitrogen from aqueous solution on chemically activated biochar prepared from sorghum distillers grain. Appl Sci 9:5249
Braghiroli FL, Bouafif H, Neculita CM et al (2019) Performance of physically and chemically activated biochars in copper removal from contaminated mine effluents. Water Air Soil Pollut 230:178
Yin Q, Ren H, Wang R et al (2018) Evaluation of nitrate and phosphate adsorption on Al-modified biochar: influence of Al content. Sci Total Environ 631:895–903
** J, Li S, Peng X, Liu W, Zhang C, Yang Y, Han L, du Z, Sun K, Wang X (2018) HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresour Technol 256:247–253
Liu P, Liu WJ, Jiang H, Chen JJ, Li WW, Yu HQ (2012) Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour Technol 121:235–240
Rajapaksha AU, Chen SS, Tsang DC et al (2016) Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 148:276–291
Ippolito JA, Ducey TF, Cantrell KB, Novak JM, Lentz RD (2016) Designer, acidic biochar influences calcareous soil characteristics. Chemosphere 142:184–191
Peng P, Lang YH, Wang XM (2016) Adsorption behavior and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms. Ecol Eng 90:225–233
Cao L, Iris KM, Tsang DC et al (2018) Phosphoric acid-activated wood biochar for catalytic conversion of starch-rich food waste into glucose and 5-hydroxymethylfurfural. Bioresour Technol 267:242–248
Cordero-Lanzac T, Hita I, Veloso A, Arandes JM, Rodríguez-Mirasol J, Bilbao J, Cordero T, Castaño P (2017) Characterization and controlled combustion of carbonaceous deactivating species deposited on an activated carbon-based catalyst. Chem Eng J 327:454–464
Hita I, Cordero-Lanzac T, Gallardo A, Arandes JM, Rodríguez-Mirasol J, Bilbao J, Cordero T, Castaño P (2016) Phosphorus-containing activated carbon as acid support in a bifunctional Pt–Pd catalyst for tire oil hydrocracking. Catal Commun 78:48–51
García-Mateos FJ, Berenguer R, Valero-Romero MJ, Rodríguez-Mirasol J, Cordero T (2018) Phosphorus functionalization for the rapid preparation of highly nanoporous submicron-diameter carbon fibers by electrospinning of lignin solutions. J Mater Chem A 6:1219–1233
Peng H, Gao P, Chu G et al (2017) Enhanced adsorption of Cu (II) and Cd (II) by phosphoric acid-modified biochars. Environ Pollut 229:846–853
Iriarte-Velasco U, Sierra I, Zudaire L et al (2016) Preparation of a porous biochar from the acid activation of pork bones. Food Bioprod Process 98:341–353
Lei S, Dongmei C, Shungang W, Zebin Y et al (2015) Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour Technol 198:300–308
Sun K, Tang J, Gong Y et al (2015) Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ Sci Pollut Res 22:16640–16651
Zhang X, Zhang L, Li A (2018) Eucalyptus sawdust derived biochar generated by combining the hydrothermal carbonization and low concentration KOH modification for hexavalent chromium removal. J Environ Manag 206:989–998
Cazetta (2011)
Mosa AA, El-Ghamry A, Al-Zahrani H et al (2017) Chemically modified biochar derived from cotton stalks: characterization And assessing its potential for heavy metals removal from wastewater. Environ Biodiv Soil Secur 1:33–45
Hu X, Xue Y, Long L et al (2018) Characteristics and batch experiments of acid-and alkali-modified corncob biomass for nitrate removal from aqueous solution. Environ Sci Pollut Res 25:19932–19940
Hasnan FI, Iamail KN, Musa M et al (2018) Characterization of bio char derived from tapioca skin. In: IOP Conference Series: Materials Science and Engineering, vol 334, pp 012–016
Shen Y, Zhang N (2019) Facile synthesis of porous carbons from silica-rich rice husk char for volatile organic compounds (VOCs) sorption. Bioresour Technol 282:294–300
Trakal L, Vítková M, Hudcová B et al (2019) Biochar and its composites for metal (loid) removal from aqueous solutions. In: Biochar from Biomass and Waste, pp 113–141
Wang S, Gao B, Zimmerman AR et al (2015) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol 175:391–395
Wang P, Tang L, Wei X et al (2017) Synthesis and application of iron and zinc doped biochar for removal of p-nitrophenol in wastewater and assessment of the influence of co-existed Pb (II). Appl Surf Sci 392:391–401
Liang J, Fang Y, Luo Y et al (2019) Magnetic nanoferromanganese oxides modified biochar derived from pine sawdust for adsorption of tetracycline hydrochloride. Environ Sci Pollut Res 26:5892–5903
Tang N, Niu C, Li X et al (2018) Efficient removal of Cd2+ and Pb2+ from aqueous solution with amino- and thiol-functionalized activated carbon: isotherm and kinetics modeling. Sci Total Environ 635:1331–1344
Akgül G, Maden TB, Diaz E et al (2019) Modification of tea biochar with Mg, Fe, Mn and Al salts for efficient sorption of PO43− and Cd2+ from aqueous solutions. J Water Reuse Desalin 9:57–66
Wang Z, Wu J, He T et al (2014) Corn stalks char from fast pyrolysis as precursor material for preparation of activated carbon in fluidized bed reactor. Bioresour Technol 167:551–554
Niksiar A, Nasernejad B (2017) Activated carbon preparation from pistachio shell pyrolysis and gasification in a spouted bed reactor. Biomass Bioenergy 106:43–50
Carrier M, Hardie AG, Uras U et al (2012) Production of char from vacuum pyrolysis of South-African sugar cane bagasse and its characterization as activated carbon and biochar. J Anal Appl Pyrolysis 96:24–32
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew Sust Energ Rev 55:467–481
Brown AB. Development and utilization of a Raman characterization method for Hydrothermal char 2019
Jiang SF, Sheng GP, Jiang H (2019) Advances in the characterization methods of biomass pyrolysis products. ACS Sustain Chem Eng 7:12639–12655
Wang J, Liao Z, Ifthikar J et al (2017) One-step preparation and application of magnetic sludge-derived biochar on acid orange 7 removal via both adsorption and persulfate based oxidation. RSC Adv 7:18696–18706
Yoo S, Kelley S, Tilotta D et al (2018) Structural characterization of loblolly pine derived biochar by x-ray diffraction and electron energy loss spectroscopy. ACS Sustain Chem Eng 6:2621–2629
Zhao Y, Feng D, Zhang Y et al (2016) Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar. Fuel Process Technol 141:54–60
Igalavithana AD, Mandal S, Niazi NK et al (2017) Advances and future directions of biochar characterization methods and applications. Crit Rev Environ Sci Technol 47:2275–2330
Intani K, Latif S, Cao Z et al (2018) Characterisation of biochar from maize residues produced in a self-purging pyrolysis reactor. Bioresour Technol 265:224–235
Shim T, Yoo J, Ryu C et al (2015) Effect of steam activation of biochar produced from a giant Miscanthus on copper sorption and toxicity. Bioresour Technol 197:85–90
Rajapaksha AU, Vithanage M, Lee SS et al (2016) Steam activation of biochars facilitates kinetics and pH-resilience of sulfamethazine sorption. J Soils Sediments 16:889–895.103
Wang RZ, Huang DL, Liu YG et al (2019) Synergistic removal of copper and tetracycline from aqueous solution by steam-activated bamboo-derived biochar. J Hazard Mater 384:121470
Franciski MA, Peres EC, Godinho M et al (2018) Development of CO2 activated biochar from solid wastes of a beer industry and its application for methylene blue adsorption. Waste Manag 78:630–638
Vaughn SF, Kenar JA, Tisserat B et al (2017) Chemical and physical properties of Paulownia elongata biochar modified with oxidants for horticultural applications. Ind Crop Prod 97:260–267
Taskin MB, Kadioglu YK, Sahin O et al (2019) Effect of acid modified biochar on the growth and essential and non-essential element content of bean, chickpea, soybean, and maize grown in calcareous soil. Commun Soil Sci Plant Anal 1–10:105
Huff MD, Lee JW (2016) Biochar-surface oxygenation with hydrogen peroxide. J Environ Manag 165:17–21
Güzel F, Sayğılı H, Sayğılı GA et al (2017) Optimal oxidation with nitric acid of biochar derived from pyrolysis of weeds and its application in removal of hazardous dye methylene blue from aqueous solution. J Clean Prod 144:260–265
Zhang D, Li Y, Sun A et al (2019) Enhanced nitrobenzene reduction by modified biochar supported sulfidated nano zerovalent iron: comparison of surface modification methods. Sci Total Environ 694:133701
El-Khateeb A, Al-Zahrani H, Selim EM et al (2017) Chemically modified biochar derived from cotton stalks: characterization and assessing its potential for heavy metals removal from wastewater. electronic effects. Water Res 80:179–188
Ding Z, Hu X, Wan Y et al (2016) Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: batch and column tests. J Ind Eng Chem 33:239–245
** HM, Capared S, Chang ZZ et al (2014) Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: sorption property and its improvement with KOH activation. Bioresour Technol 169:622–629
Li B, Yang L, Wang CQ et al (2017) Adsorption of Cd (II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 175:332–340
He R, Yuan X, Huang Z et al (2019) Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surf A Physicochem Eng Asp 579:123642
Dehkhoda AM, Gyenge E, Ellis N et al (2016) A novel method to tailor the porous structure of KOH-activated biochar and its application in capacitive deionization and energy storage. Biomass Bioenergy 87:107–121
Ding S, Liu Y (2020) Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 260:116–382
Duan XL, Yuan CG, **g TT et al (2019) Removal of elemental mercury using large surface area micro-porous corn cob activated carbon by zinc chloride activation. Fuel 239:830–840
Park JH, Ok YS, Kim SH et al (2015) Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142:77–83
Naeem MA, Imran M, Amjad M (2019) Batch and column scale removal of cadmium from water using raw and acid activated wheat straw biochar. Water 11:1438
Zhu L, Zhao N, Tong L (2018) Structural and adsorption characteristics of potassium carbonate activated biochar. RSC Adv 8:21012–21019
Jung C, Boateng LK, Flora JR et al (2015) Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: experimental and molecular modeling study. Chem Eng J 264:1–9
Li C, Zhang L, Gao Y et al (2018) Facile synthesis of nano ZnO/ZnS modified biochar by directly pyrolyzing of zinc contaminated corn stover for Pb (II), Cu (II) and Cr (VI) removals. Waste Manag 79:625–637
Wang S, Gao B, Li Y et al (2015) Manganese oxide-modified biochars: preparation, characterization, and sorption of arsenate and lead. Bioresour Technol 181:13–17
Gan C, Liu Y, Tan X et al (2015) Effect of porous zinc–biochar nanocomposites on Cr (VI) adsorption from aqueous solution. RSC Adv 5:35107–35115
Wang H, Gao B, Wang S et al (2015) Removal of Pb (II), Cu (II), and Cd (II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour Technol 197:356–362
Trakal L, Veselská V, Šafařík I et al (2016) Lead and cadmium sorption mechanisms on magnetically modified biochars. Bioresour Technol 203:318–324
Fan Z, Zhang Q, Li M et al (2018) Investigating the sorption behavior of cadmium from aqueous solution by potassium permanganate-modified biochar: quantify mechanism and evaluate the modification method. Environ Sci Pollut Res 25:8330–8339
Zhou L, Huang Y, Qiu W et al (2017) Adsorption properties of nano-MnO2–biochar composites for copper in aqueous solution. Molecules 22:173
Jiang D, Chu B, Amano Y et al (2018) Removal and recovery of phosphate from water by Mg-laden biochar: batch and column studies. Colloids Surf A Physicochem Eng Asp 558:429–437
Li H, Dong X, da Silva EB et al (2017) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466–478
Yu J, Jiang C, Guan Q et al (2018) Enhanced removal of Cr (VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere 195:632–640
Han Y, Cao X, Ouyang X et al (2016) Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: effects of production conditions and particle size. Chemosphere 145:336–341
Li R, Wang JJ, Zhou B et al (2017) Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J Clean Prod 147:96–107
O'Connor D, Peng T, Li G et al (2018) Sulfur-modified rice husk biochar: a green method for the remediation of mercury contaminated soil. Sci Total Environ 621:819–826
Villalobos M, Escobar-Quiroz IN, Salazar-Camacho C (2014) The influence of particle size and structure on the sorption and oxidation behavior of birnessite: I. Adsorption of As (V) and oxidation of As (III). Geochim Cosmochim Acta 125:564–581
Yu Z, Zhou L, Huang Y et alEffects of a manganese oxide-modified biochar composite on adsorption of arsenic in red soil. J Environ Manag 163:155–162
Zhang F, Wang X, **onghui J et al (2016) Efficient arsenate removal by magnetite-modified water hyacinth biochar. Environ Pollut 216:575–583
Lu K, Yang X, Gielen G et al (2017) Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J Environ Manag 186:285–292
Liu H, Xu F, **e Y et al (2018) Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci Total Environ 645:702–709
Frick H, Tardif S, Kandeler E et al (2019) Assessment of biochar and zero-valent iron for in-situ remediation of chromated copper arsenate contaminated soil. Sci Total Environ 655:414–422
Moon DH, Hwang I, Chang YY et al (2017) Quality improvement of acidic soils by biochar derived from renewable materials. Environ Sci Pollut Res 24:4194–4199
Lin L, Li Z, Liu X et al (2019) Effects of Fe-Mn modified biochar composite treatment on the properties of As-polluted paddy soil. Environ Pollut 244:600–607
Cerino-Córdova FJ, Díaz-Flores PE, García-Reyes RB et al (2013) Biosorption of Cu (II) and Pb (II) from aqueous solutions by chemically modified spent coffee grains. Int J Environ Sci Technol 10:611–622
Yan L, Kong L, Qu Z et al (2015) Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sustain Chem Eng 3:125–132
Agrafioti E, Kalderis D, Diamadopoulos E (2014) Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions. J Environ Manag 146:444–450
Son EB, Poo KM, Chang JS et al (2018) Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci Total Environ 615:161–168
Li Y, Shao J, Wang X et al (2014) Characterization of modified biochars derived from bamboo pyrolysis and their utilization for target component (furfural) adsorption. Energy Fuel 28:5119–5127
Sun L, Chen D, Wan S et al (2015) Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour Technol 198:300–308
Chen B, Chen Z, Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol 102:716–723
Ahmad M, Lee SS, Rajapaksha AU et al (2013) Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour Technol 143:615–622
Zhang XN, Mao GY, Jiao YB et al (2014) Adsorption of anionic dye on magnesium hydroxide-coated pyrolytic bio-char and reuse by microwave irradiation. Int J Environ Sci Technol 11:1439–1448
Mubarak NM, Kundu A, Sahu JN et al (2014) Synthesis of palm oil empty fruit bunch magnetic pyrolytic char impregnating with FeCl3 by microwave heating technique. Biomass Bioenergy 61:265–275
Wu JW, Wu CR, Zhou CS et al (2020) Fate and removal of antibiotic resistance genes in heavy metals and dye co-contaminated wastewater treatment system amended with β-cyclodextrin functionalized biochar. Sci Total Environ 723:1–8
**g XR, Wang YY, Liu WJ et al (2014) Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem Eng J 248:168–174
Lee J, Kim KH, Kwon EE (2017) Biochar as a catalyst. Renew Sust Energ Rev 77:70–79
Zhang X, Tong J, Hu BX et al (2018) Adsorption and desorption for dynamics transport of hexavalent chromium (Cr (VI)) in soil column. Environ Sci Pollut Res 25:459–468
Tian K, Liu WJ, Zhang S et al (2016) One-pot synthesis of a carbon supported bimetallic Cu–Ag NPs catalyst for robust catalytic hydroxylation of benzene to phenol by fast pyrolysis of biomass waste. Green Chem 18:5643–5650
Zhao C, Lv P, Yang L et al (2018) Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers Manag 160:477–485
Zhao C, Yang L, **ng S et al (2018) Biodiesel production by a highly effective renewable catalyst from pyrolytic rice husk. J Clean Prod 199:772–780
Shen Y, Zhao P, Shao Q et al (2014) In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl Catal B Environ 152:140–151
Swierczynski D, Libs S, Courson C et al (2007) Steam reforming of tar from a biomass gasification process over Ni/olivine catalyst using toluene as a model compound. Appl Catal B Environ 74:211–222
Muradov N, Fidalgo B, Gujar AC et al (2012) Production and characterization of Lemna minor bio-char and its catalytic application for biogas reforming. Biomass Bioenergy 42:123–131
Wang L, Wang Y, Ma F et al (2019) Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: a review. Sci Total Environ 668:1298–1309
Qian K, Kumar A, Zhang H et al (2015) Recent advances in utilization of biochar. Renew Sust Energ Rev 42:1055–1064
Jiang J, Zhang L, Wang X et al (2013) Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes. Electrochim Acta 113:481–489
He N, Yoo S, Meng J et al (2017) Engineering biorefinery residues from loblolly pine for supercapacitor applications. Carbon. 120:304–312
Wang Y, Wang Y, Jiang L (2018) Freestanding carbon aerogels produced from bacterial cellulose and its Ni/MnO 2/Ni (OH) 2 decoration for supercapacitor electrodes. J Appl Electrochem 48:495–507
Jiang C, Yakaboylu GA, Yumak T et al (2020) Activated carbons prepared by indirect and direct CO2 activation of lignocellulosic biomass for supercapacitor electrodes. Renew Energy 20:1–41
Yu J, Zhao Y, Li Y (2014) Utilization of corn cob biochar in a direct carbon fuel cell. J Power Sources 270:312–317
**ong X, Iris KM, Cao L et al (2017) A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour Technol 246:254–270
Kacprzak A, Kobyłecki R, Włodarczyk R et al (2014) The effect of fuel type on the performance of a direct carbon fuel cell with molten alkaline electrolyte. J Power Sources 255:179–186
Acknowledgements
The authors are grateful to Indian Council of Agricultural Research (ICAR), Govt. of India for providing financial support to design and development of vacuum pyrolysis reactor for activated biochar production under Consortium Research Platform on Energy from Agriculture (CRP on EA). The author (Ashish Pawar) is also thankful to Council for Scientific and Industrial Research (CSIR), Govt. of India for providing research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panwar, N.L., Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: a review. Biomass Conv. Bioref. 12, 925–947 (2022). https://doi.org/10.1007/s13399-020-00870-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00870-3