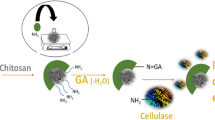
Abstract
Cellulases convert lignocellulosic biomass into fermentable sugars which further act as substrate for ethanol production. Our previous research was focused on enzymatic hydrolysis of rice straw by free cellulases for production of fermentable sugars. Immobilization is a powerful tool to increase the stability and reusability of cellulases besides improving the economy of ethanol production process from rice straw. In the present study, cellulase produced from Aspergillus fumigatus was immobilized on magnetic nanoparticles by using glutaraldehyde cross linker with a binding efficiency of 65.55%. The electron microscopy and spectroscopy tools confirmed the enzyme immobilization process on magnetic nanoparticles. The immobilized cellulase exhibited filter paper, carboxymethyl cellulase and cellobiase activities of 11.82, 21.36 and 10.81 IU, respectively. The free and immobilized cellulase exhibited identical pH optima (pH 5.0) while different temperature optima of 50 °C and 60 °C, respectively. The immobilized enzyme retained 56.87% of its maximal activity after 6 h of pre-incubation at 60 °C. Km (Michaelis constant) and Vmax (maximum velocity) of immobilized enzyme were 11.76 mM and 1.17 μmol min−1 ml−1, respectively. The immobilized cellulase hydrolysed pre-treated rice straw with saccharification efficiency of 52.67%. Further, it could be reutilized for up to four saccharification cycles with retention of 50.34% activity. Therefore, the improved properties of magnetic nanoparticle-immobilized cellulase and its reusability benefits offer a promising potential for industrial production of fermentable sugars and ethanol from rice straw.

Graphical abstract












Similar content being viewed by others
References
Wyman CE, Cai CM, Kumar R (2019) Bioethanol from lignocellulosic biomass. In: Kaltschmitt M (ed) Energy from organic materials (biomass) a volume in the encyclopedia of sustainability science and technology, 2nd edn. Springer, New York, pp 997–1022
Zhang Z, Liu B, Zhao ZK (2012) Efficient acid catalyzed hydrolysis of cellulose in organic electrolyte solutions. Polym Degrad Stab 97:573–577
Kuila A, Sharma V, Garlapati VK, Singh A, Roy L, Banerjee R (2016) Present statu s on enzymatic hydrolysis of lignocellulosic biomass for bioethanol production. Adv Biofeedstocks Biofuels 1:85
Brummer V, Jurena T, Hlavacek V, Omelkova J, Bebar L, Gabriel P, Stehlik P (2014) Enzymatic hydrolysis of pretreated waste paper–source of raw material for production of liquid biofuels. Bioresour Technol 152:543–547
Madadi M, Tu Y, Abbas A (2017) Recent status on enzymatic saccharification of lignocellulosic biomass for bioethanol production. Electron J Biol 13:135–143
Ahamed A, Vermette P (2008) Culture based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-30 in bioreactor culture conditions. Biochem Eng 140:399–407
Zhang Q, Han X, Tang B (2013) Preparation of a magnetically recoverable biocatalyst support on monodisperse Fe3O4 nanoparticles. RSC Adv 3:9924–9931
Chundawat S, Sousa LDC, Cheh AM, Balan V, Dale B (2017) Methods for producing extracted and digested products from pretreated lignocellulosic biomass. U.S. patent, 9650657
Mitchell DT, Lee SB, Trofin L, Li N, Nevanen TK, Soderlund H, Martin CR (2002) Smart nanotubes for bioseparations and biocatalysis. J Am Chem Soc 124:11864–11865
Eldin MSM (2016) Enzyme immobilization: nanopolymers for enzyme immobilization applications. Energy 16:18
Huang WC, Wang W, Xue C, Mao X (2018) Effective enzyme immobilization onto a magnetic chitin nanofiber composite. ACS Sustain Chem Eng 6:8118–8124
Ahmad R, Khare SK (2018) Immobilization of Aspergillus niger cellulase on multiwall carbon nanotubes for cellulose hydrolysis. Bioresour Technol 252:72–75
Otari SV, Patel SK, Kim SY, Haw JR, Kalia VC, Kim IW, Lee JK (2019) Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian J Microbiol 59:105–108
Abbaszadeh M, Hejazi P (2019) Metal affinity immobilization of cellulase on Fe3O4 nanoparticles with copper as ligand for biocatalytic applications. Food Chem 290:47–55
Verma ML, Barrow CJ, Puri M (2013) Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Appl Microbiol Biotechnol 97:23–39
Kim K, Lee OK, Lee E (2018) Nano-immobilized biocatalysts for biodiesel production from renewable and sustainable resources. J Catal 8:68
García PF, Brammen M, Wolf M, Reinlein S, von Roman MF, Berensmeier S (2015) High-gradient magnetic separation for technical scale protein recovery using low cost magnetic nanoparticles. Sep Purif Technol 150:29–36
Kumar A, Singh S, Nain L (2018) Magnetic nanoparticle immobilized cellulase enzyme for saccharification of paddy straw. Int J Curr Microbiol App Sci 7:881–893
Abraham RE, Verma ML, Barrow CJ, Puri M (2014) Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol Biofuels 7:90
Selvam K, Govarthanan M, Senbagam D, Kamala-Kannan S, Senthilkumar B, Selvankumar T (2016) Activity and stability of bacterial cellulase immobilized on magnetic nanoparticles. Chin J Catal 37:1891–1898
**ao A, Xu C, Lin Y, Ni H, Zhu Y, Cai H (2016) Preparation and characterization of κ-carrageenase immobilized onto magnetic iron oxide nanoparticles. Electron J Biotechnol 19:1–7
Han J, Luo P, Wang Y, Wang L, Li C, Zhang W, Dong J, Ni L (2018) The development of nanobiocatalysis via the immobilization of cellulase on composite magnetic nanomaterial for enhanced loading capacity and catalytic activity. Int J Biol Macromol 119:692–700
Dong RJ, Zheng DF, Yang DJ, Qiu XQ (2019) pH-responsive lignin-based magnetic nanoparticles for recovery of cellulase. Bioresour Technol 294:122133
Kumar A, Singh S, Tiwari R, Goel R, Nain L (2017) Immobilization of indigenous holocellulase on iron oxide (Fe2O3) nanoparticles enhanced hydrolysis of alkali pretreated paddy straw. Int J Biol Macromol 96:538–549
Zhang Q, Kang J, Yang B, Zhao L, Hou Z, Tang B (2016) Immobilized cellulase on Fe3O4 nanoparticles as a magnetically recoverable biocatalyst for the decomposition of corncob. Chin J Catal 37:389–397
Manasa P, Saroj P, Korrapati N (2017) Immobilization of cellulase enzyme on zinc ferrite nanoparticles in increasing enzymatic hydrolysis on ultrasound assisted alkaline pretreated Crotalaria juncea biomass. Indian J Sci Technol 10:1–7
Poorakbar E, Shafiee A, Saboury AA, Rad BL, Khoshnevisan K, Ma'mani L, Derakhshankhah H, Ganjali MR, Hosseini M (2018) Synthesis of magnetic gold mesoporous silica nanoparticles core shell for cellulase enzyme immobilization: improvement of enzymatic activity and thermal stability. Process Biochem 71:92–100
Binod P, Sindhu R, Singhania RR, Vikram S, Devi L, Nagalakshmi S, Kurien N, Sukumaran RK, Pandey A (2010) Bioethanol production from rice straw: an overview. Bioresour Technol 101:4767–4774
Raj T, Kapoor M, Gaur R, Christopher J, Lamba BY, Tuli DK, Kumar R (2015) Physical and chemical characterization of various Indian agriculture residues for biofuels production. Energ Fuels 29:3111–3118
Colonia BSO, Junior AFC (2014) Screening and detection of extracellular cellulases (endo-and exo-glucanases) secreted by filamentous fungi isolated from soils using rapid tests with chromogenic dyes. Afr J Biotechnol 13:4694–4701
Meyyappan A, Shakila BA, Kurian GA (2015) One step synthesis of iron oxide nanoparticles via chemical and green route- an effective comparison. Int J Pharm Pharm Sci 7:70–74
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mandels M, Andreotti R, Roche C (1976) Measurement of saccharifying cellulase. Biotechnol Bioeng Symp. Pp 21-33. US Army Natick development center, Nauck, MA
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods Enzymol 160:87–112
Toyama M, Ogawa K (1977) Cellulase production of Trichoderma viride in solid and submerged culture methods. In: Ghose TK (ed) Proc bioconversion of cellulosic substances into energy, chemicals and microbial protein, vol 1. IIT, New Delhi, pp 305–327
Xu J, Huo S, Yuan Z, Zhang Y, Xu H, Guo Y, Liang C, Zhuang X (2011) Characterization of direct cellulase immobilization with superparamagnetic nanoparticles. Biocatal Biotransform 29:71–76
Lu PJ, Fu WE, Huang SC, Lin CY, Ho ML, Chen YP, Cheng HF (2018) Methodology for sample preparation and size measurement of commercial ZnO nanoparticles. J Food Drug Anal 26:628–636
Predoi D (2007) A study on iron oxide nanoparticles coated with dextrin obtained by coprecipitation. Dig J Nanomater Bios 2:169–173
Burgula Y, Khali D, Kim S, Krishnan SS, Cousin MA, Gore JP, Reuhs BL, Mauer LJ (2006) Detection of Escherichia coli O157:H7 and Salmonella typhimurium using filtration followed by Fourier-transform infrared spectroscopy. J Food Prot 69:1777–1784
Kaur P, Kocher GS, Taggar MS, Sooch SS, Kumar V (2017) Assessment of thermophilic fungal strains for cellulase production using chemical pretreated rice straw. Chem Sci Rev Lett 6:629–634
Crompton EW, Maynard LA (1938) The relation of cellulose and lignin content to the nutritive value of animal feeds. J Anim Nut 15:391–392
Goering HK, Van Soest PJ (1970) Forage fibre analysis. Pp. 1-20. Agricultural research services, Washington DC, U.S.
AOAC (2000) Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Washington, D.C.
Singh A, Tuteja S, Singh N, Bishnoi NR (2011) Enhanced saccharification of rice straw and hull by microwave-alkali pre-treatment and lignocellulolytic enzyme production. Bioresour Technol 102:1773–1782
Jia J, Zhang W, Yang Z, Yang X, Wang N, Yu X (2017) Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules 22:269
Mohamed SA, Al-Harbi MH, Almulaiky YQ, Ibrahim IH, El-Shishtawy RM (2017) Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron J Biotechnol 27:84–90
Kouassi GK, Irudayaraj J, McCarty G (2005) Examination of cholesterol oxidase attachment to magnetic nanoparticles. J Nanobiotechnol 3:1
Can HK, Kavlak S, Parvizi Khosroshahi S, Güner A (2018) Preparation, characterization and dynamical mechanical properties of dextran-coated iron oxide nanoparticles (DIONPs). Artif Cells Nanomed Biotechnol 46:421–431
Chittur KK (1998) FTIR/ATR for protein adsorption to biomaterial surfaces. Biomater 19:357–369
Herzberg G, Crawford BL Jr (1946) Infrared and Raman spectra of polyatomic molecules. J Phys Chem 50:288
Jordan J, Kumar CS, Theegala C (2011) Preparation and characterization of cellulase-bound magnetite nanoparticles. J Mol Catal B Enzym 68:139–146
Khoshnevisan K, Bordbar AK, Zare D, Davoodi D, Noruzi M, Barkhi M, Tabatabaei M (2011) Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem Eng J 171:669–673
Tao QL, Li Y, Shi Y, Liu RJ, Zhang YW, Guo J (2016) Application of molecular imprinted magnetic Fe3O4@SiO2 nanoparticles for selective immobilization of cellulase. J Nanosci Nanotechnol 16:6055–6060
Tu M, Zhang X, Kurabi A, Gilkes N, Mabee W, Saddler J (2006) Immobilization of β-glucosidase on Eupergit C for lignocellulose hydrolysis. Biotechnol Lett 28:151–156
Weetall HH (1993) Preparation of immobilized proteins covalently coupled through silane coupling agents to inorganic supports. Appl Biochem Biotechnol 41:157–188
Brodeur G, Yau E, Badal K, Collier J, Ramachandran KB, Ramakrishnan S (2011) Chemical and physiochemical pretreatment of lignocellulosic biomass: a review. Enzym Res 2011:782532
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18
Weerasai K, Suriyachai N, Poonsrisawat A, Arnthong J, Unrean P, Laosiripojana N, Champreda V (2014) Sequential acid and alkaline pretreatment of rice straw for bioethanol fermentation. BioResources 9:5988–6001
Syazwanee MGMF, Shaziera AN, Izzati MZNA, Azwady AN, Muskhazli A (2018) Improvement of delignification, desilication and cellulosic content availability in paddy straw via physico-chemical pretreatments. Annu Res Rev Biol 6:1–11
Huang PJ, Chang KL, Hsieh JF, Chen ST (2015) Catalysis of rice straw hydrolysis by the combination of immobilized cellulase from Aspergillus niger on β-cyclodextrin-Fe3O4 nanoparticles and ionic liquid. Biomed Res Int 2015:409103
Baskar G, Kumar RN, Melvin XH, Aiswarya R, Soumya S (2016) Sesbania aculeate biomass hydrolysis using magnetic nanobiocomposite of cellulase for bioethanol production. Renew Energy 98:23–28
Periyasamy K, Santhalembi L, Mortha G, Aurousseau M, Boyer A, Subramanian S (2018) Bioconversion of lignocellulosic biomass to fermentable sugars by immobilized magnetic cellulolytic enzyme cocktails. Langmuir 34:6546–6555
Acknowledgements
Authors are thankful to Department of Renewable Energy Engineering, Punjab Agricultural University, Ludhiana, Punjab, India, for providing the necessary facilities to undertake this study. Authors are thankful to Sophisticated Analytical Instrumentation Facility, Panjab University, Chandigarh, Punjab, India, for providing instrumentation for analysis of sample by SEM, EDS and XRD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, P., Taggar, M.S. & Kalia, A. Characterization of magnetic nanoparticle–immobilized cellulases for enzymatic saccharification of rice straw. Biomass Conv. Bioref. 11, 955–969 (2021). https://doi.org/10.1007/s13399-020-00628-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00628-x