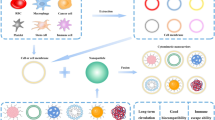
Abstract
The global prevalence of cancer is increasing, necessitating new additions to traditional treatments and diagnoses to address shortcomings such as ineffectiveness, complications, and high cost. In this context, nano and microparticulate carriers stand out due to their unique properties such as controlled release, higher bioavailability, and lower toxicity. Despite their popularity, they face several challenges including rapid liver uptake, low chemical stability in blood circulation, immunogenicity concerns, and acute adverse effects. Cell-mediated delivery systems are important topics to research because of their biocompatibility, biodegradability, prolonged delivery, high loading capacity, and targeted drug delivery capabilities. To date, a variety of cells including blood, immune, cancer, and stem cells, sperm, and bacteria have been combined with nanoparticles to develop efficient targeted cancer delivery or diagnosis systems. The review paper aimed to provide an overview of the potential applications of cell-based delivery systems in cancer therapy and diagnosis.
Graphical abstract












Similar content being viewed by others
Data availability
All required data are available in the manuscript.
Abbreviations
- 5-FU:
-
5-Fluorouracil
- AF-SPNPs:
-
Activated fibroblasts cell membrane modified SPNPs
- AA:
-
Acrylic acid
- ASCs:
-
Adult stem cells
- ADSCs:
-
Adipose-derived stem cells
- AuNR:
-
Au nanorod
- AuNRB:
-
Au nanorod-modified with bovine serum albumin
- AuNRBR:
-
Au Nanorod-modified with bovine serum albumin functionalized with RGD Peptide
- APCs:
-
Antigen-presenting cells
- Apdl1:
-
Anti-Pdl1
- Anti-Tnfα:
-
Anti-tumor necrosis factor
- BOMVs:
-
Bacteria-derived outer membrane vesicles
- BFGF:
-
Basic fibroblast growth factor
- BH:
-
Berberine hydrochloride
- BBB:
-
Blood brain barrier
- BTICs:
-
Brain tumor-initiating cells
- CAAs:
-
Cancer-associated adipocytes
- CCM:
-
Cancer cell membrane
- CC-UPCNPs:
-
Cancer cell membrane modified UCNPs
- CAR:
-
Chimeric antigen receptor
- CTCs:
-
Circulating tumor cells
- Cur:
-
Curcumin
- CD:
-
Cytosine deaminase
- CDase:
-
Cytosine deaminase
- CTLs:
-
Cytotoxic T-lymphocytes
- CMV:
-
Cytomegalovirus
- DCs:
-
Dendritic cells
- DSN:
-
Double-specific nucleases
- DOX:
-
Doxorubicin
- FA-UCNPs:
-
DSPE-PEG-FA-coated UCNPs
- EPR:
-
Enhanced permeability and retention
- EVs:
-
Extracellular vesicles
- FLI:
-
Fluorescence imaging
- GCB:
-
Gemcitabine
- GinPa:
-
Gingival papilla
- GA:
-
Glutaraldehyde
- AuNCs:
-
Gold nanocages
- IFN-β:
-
Human interferon-β
- HNSCs:
-
Human neural stem cells
- ICG:
-
Indocyanine green
- LAMP:
-
Lysosomal associated membrane protein
- MRI:
-
Magnetic resonance imaging
- MSCs:
-
Mesenchymal stem cells
- MSNs:
-
Mesoporous silica nanoparticles
- MOFs:
-
Metal-organic frameworks
- MTX-liposome:
-
Methotrexate-encapsulated liposomes
- MTX-Liposome:
-
Methotrexate-encapsulated liposomes
- NCs:
-
Nanocarries
- NPs:
-
Nanoparticles
- NIR:
-
Near-infrared
- OMVs:
-
Outer membrane vesicles
- PTX:
-
Paclitaxel
- PEG:
-
Polyethylene glycol
- PEGylated AuNPs:
-
Polyethylene glycol-coated Au NPs
- PFCs:
-
Perfluorocarbons
- PAI:
-
Photoacoustic imaging
- PDT:
-
Photodynamic therapy
- PTT:
-
Photothermal therapy
- PMDVs:
-
Platelet membrane-derived vesicles
- PMs:
-
Platelet membranes
- PCLP1:
-
Podocalyxin-like protein 1
- PVP:
-
Poly vinylpyrrolidone
- PCL:
-
Poly(caprolactone)
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PB:
-
Prussian blue
- RENMs:
-
Rare earth dope nanomaterial
- RBC-MNs:
-
RBCs membrane coated iron oxide magnetic nanoparticles
- RBCM:
-
Red blood cell membranes
- RBCs:
-
Red blood cells
- RES:
-
Reticuloendothelial system
- MSCVs:
-
Mesenchymal stem cell membrane vesicles
- SPNPs:
-
Semiconducting polymer NPs
- SCs:
-
Stem cells
- St:
-
Styrene
- SPIONs:
-
Super magnetic iron oxide nanoparticles
- ITP:
-
Thrombocytopenia
- TLR4:
-
Toll-like receptor 4
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- TCIPA:
-
Tumor cell-induced platelet aggregation
- TICs:
-
Tumor-infiltrating immune cells
- UCNPs:
-
Up conversion nanoparticles
- WBCs:
-
White blood cells
References
Alimardani V, Abolmaali SS, Tamaddon AM, Ashfaq M. Recent advances on microneedle arrays-mediated technology in cancer diagnosis and therapy. Drug Del Transl Res. 2020:1–29.
Li T, Dong H, Zhang C, Mo R. Cell-based drug delivery systems for biomedical applications. Nano Res. 2018;11(10):5240–57.
Abolmaali S, Tamaddon A, Salmanpour M, Mohammadi S, Dinarvand R. Block ionomer micellar nanoparticles from double hydrophilic copolymers, classifications and promises for delivery of cancer chemotherapeutics. Eur J Pharm Sci. 2017;104:393–405.
Taghizadeh S, Alimardani V, Roudbali PL, Ghasemi Y, Kaviani E. 1Gold nanoparticles application in liver cancer. Photodiagnosis Photodyn Ther. 2019.
Alimardani V, Abolmaali SS, Yousefi G, Rahiminezhad Z, Abedi M, Tamaddon A, et al. Microneedle arrays combined with nanomedicine approaches for transdermal delivery of therapeutics. J Clin Med. 2021;10(2):181.
Ardakani LS, Alimardani V, Tamaddon AM, Amani AM, Taghizadeh S. Green synthesis of iron-based nanoparticles using Chlorophytum comosum leaf extract: methyl orange dye degradation and antimicrobial properties. Heliyon. 2021;7(2):e0615.
Abedi M, Abolmaali SS, Heidari R, Samani SM, Tamaddon AM. Hierarchical mesoporous zinc-imidazole dicarboxylic acid MOFs: Surfactant-directed synthesis, pH-responsive degradation, and drug delivery. Int J Pharm. 2021;602:120685.
Mostoufi H, Yousefi G, Tamaddon A-m, Firuzi O. Reversing multi-drug tumor resistance to Paclitaxel by well-defined pH-sensitive amphiphilic polypeptide block copolymers via induction of lysosomal membrane permeabilization. Colloids Surf B Biointerfaces. 2019;174:17–27.
Abbasi S, Yousefi G, Tamaddon A-M. Polyacrylamide–b-copolypeptide hybrid copolymer as pH-responsive carrier for delivery of paclitaxel: Effects of copolymer composition on nanomicelles properties, loading efficiency and hemocompatibility. Colloids Surf Physicochem Eng Aspects. 2018;537:217–26.
Alimardani V, Farahavar G, Salehi S, Taghizadeh S, Ghiasi MR, Abolmaali SS. Gold nanocages in cancer diagnosis, therapy, and theranostics: a brief review. Front Mater Sci. 2021:1–18.
Wei Y, Gu X, Sun Y, Meng F, Storm G, Zhong Z. Transferrin-binding peptide functionalized polymersomes mediate targeted doxorubicin delivery to colorectal cancer in vivo. J Controlled Release. 2020.
Cherkasov VR, Mochalova EN, Babenyshev AV, Rozenberg JM, Sokolov IL, Nikitin MP. Antibody-directed metal-organic framework nanoparticles for targeted drug delivery. Acta Biomater. 2020;103:223–36.
Timin AS, Litvak MM, Gorin DA, Atochina-Vasserman EN, Atochin DN, Sukhorukov GB. Cell-based drug delivery and use of nano-and microcarriers for cell functionalization. Adv Healthcare Mater. 2018;7(3):1700818.
Borandeh S, Alimardani V, Abolmaali SS, Seppälä J. Graphene family nanomaterials in ocular applications: physicochemical properties and toxicity. Chem Res Toxicol. 2021.
Alimardani V, Abolmaali SS, Tamaddon AM. Recent advances on nanotechnology-based strategies for prevention, diagnosis, and treatment of coronavirus infections. J Nanomater. 2021;2021.
Alimardani V, Abolmaali SS, Borandeh S. Antifungal and antibacterial properties of graphene-based nanomaterials: a mini-review. J Nanostruct. 2019;9(3):402–13.
Pinelli F, Perale G, Rossi F. Coating and functionalization strategies for nanogels and nanoparticles for selective drug delivery. Gels. 2020;6(1):6.
Fam SY, Chee CF, Yong CY, Ho KL, Mariatulqabtiah AR, Tan WS. Stealth coating of nanoparticles in drug-delivery systems. Nanomaterials. 2020;10(4):787.
Banskota S, Yousefpour P, Chilkoti A. Cell-based biohybrid drug delivery systems: the best of the synthetic and natural worlds. Macromol Biosci. 2017;17(1):1600361.
Su Y, **e Z, Kim GB, Dong C, Yang J. Design strategies and applications of circulating cell-mediated drug delivery systems. ACS Biomater Sci Eng. 2015;1(4):201–17.
Kamal Z, Su J, Qiu M. Erythrocytes modified (coated) gold nanoparticles for effective drug delivery. Metal Nanoparticles Drug Deliv Diag Appl. Elsevier. 2020:13–29.
Sun Y, Su J, Liu G, Chen J, Zhang X, Zhang R, et al. Advances of blood cell-based drug delivery systems. Eur J Pharm Sci. 2017;96:115–28.
Li S, Liu J, Sun M, Wang J, Wang C, Sun Y. Cell Membrane-camouflaged nanocarriers for cancer diagnostic and therapeutic. Front Pharmacol. 2020:11.
**e Z, Su Y, Kim GB, Selvi E, Ma C, Aragon-Sanabria V, et al. Immune cell-mediated biodegradable theranostic nanoparticles for melanoma targeting and drug delivery. Small. 2017;13(10):1603121.
Yu H, Yang Z, Li F, Xu L, Sun Y. Cell-mediated targeting drugs delivery systems. Drug Deliv. 2020;27(1):1425–37.
Lee N-H, You S, Taghizadeh A, Taghizadeh M, Kim HS. Cell Membrane-cloaked nanotherapeutics for targeted drug delivery. Int J Mol Sci. 2022;23(4):2223.
Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc Natl Acad Sci. 1973;70(9):2663–6.
Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Controlled Release. 2007;118(2):145–60.
Wang L-Y, Shi X-Y, Yang C-S, Huang D-M. Versatile RBC-derived vesicles as nanoparticle vector of photosensitizers for photodynamic therapy. Nanoscale. 2013;5(1):416–21.
Cohen Y, Afri M, Frimer AA. NMR-based molecular ruler for determining the depth of intercalants within the lipid bilayer: Part II. The preparation of a molecular ruler. Chem Phys Lipids. 2008;155(2):114–9.
Tan S, Wu T, Zhang D, Zhang Z. Cell or cell membrane-based drug delivery systems. Theranostics. 2015;5(8):863.
Koleva L, Bovt E, Ataullakhanov F, Sinauridze E. Erythrocytes as carriers: from drug delivery to biosensors. Pharmaceutics. 2020;12(3):276.
Guido C, Maiorano G, Cortese B, D’Amone S, Palamà IE. Biomimetic nanocarriers for cancer target therapy. Bioengineering. 2020;7(3):111.
Zhu D-M, **e W, **ao Y-S, Suo M, Zan M-H, Liao Q-Q, et al. Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy. Nanotechnology. 2018;29(8): 084002.
**ao J, Weng J, Wen F, Ye J. Red blood cell membrane-coated silica nanoparticles codelivering DOX and ICG for effective lung cancer therapy. ACS omega. 2020.
Li C, Wang X, Li R, Yang X, Zhong Z, Dai Y, et al. Resveratrol-loaded PLGA nanoparticles functionalized with red blood cell membranes as a biomimetic delivery system for prolonged circulation time. Journal of Drug Delivery Science and Technology. 2019;54: 101369.
Zhang L, Wang Z, Zhang Y, Cao F, Dong K, Ren J, et al. Erythrocyte membrane cloaked metal–organic framework nanoparticle as biomimetic nanoreactor for starvation-activated colon cancer therapy. ACS Nano. 2018;12(10):10201–11.
Piao J-G, Wang L, Gao F, You Y-Z, **ong Y, Yang L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8(10):10414–25.
**e X, Wang H, Williams GR, Yang Y, Zheng Y, Wu J, et al. Erythrocyte membrane cloaked curcumin-loaded nanoparticles for enhanced chemotherapy. Pharmaceutics. 2019;11(9):429.
Huang J, Lai W, Wang Q, Tang Q, Hu C, Zhou M, et al. Effective triple-negative breast cancer targeted treatment using iRGD-modified RBC membrane-camouflaged nanoparticles. Int J Nanomed. 2021;16:7497.
Lu Y, Hu Q, Jiang C, Gu Z. Platelet for drug delivery. Curr Opin Biotechnol. 2019;58:81–91.
Nieswandt B, Pleines I, Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J Thromb Haemost. 2011;9:92–104.
Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nature Biomedical Engineering. 2017;1(2):1–10.
Xu P, Zuo H, Chen B, Wang R, Ahmed A, Hu Y, et al. Doxorubicin-loaded platelets as a smart drug delivery system: an improved therapy for lymphoma. Sci Rep. 2017;7:42632.
Jiménez-Jiménez C, Manzano M, Vallet-Regí M. Nanoparticles coated with cell membranes for biomedical applications. Biology. 2020;9(11):406.
Zuo H, Tao J, Shi H, He J, Zhou Z, Zhang C. Platelet-mimicking nanoparticles co-loaded with W18O49 and metformin alleviate tumor hypoxia for enhanced photodynamic therapy and photothermal therapy. Acta Biomater. 2018;80:296–307. https://doi.org/10.1016/j.actbio.2018.09.017.
Zhai Y, Su J, Ran W, Zhang P, Yin Q, Zhang Z, et al. Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics. 2017;7(10):2575.
Li J, Ai Y, Wang L, Bu P, Sharkey CC, Wu Q, et al. Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials. 2016;76:52–65.
Bang K-H, Na Y-G, Huh HW, Hwang S-J, Kim M-S, Kim M, et al. The delivery strategy of paclitaxel nanostructured lipid carrier coated with platelet membrane. Cancers (Basel). 2019;11(6):807.
Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15(11):669–82.
Huang Y, Gao X, Chen J. Leukocyte-derived biomimetic nanoparticulate drug delivery systems for cancer therapy. Acta Pharmaceutica Sinica B. 2018;8(1):4–13.
Zhang W, Wang M, Tang W, Wen R, Zhou S, Lee C, et al. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv Mater. 2018;30(50):1805557.
Lutz H, Hu S, Dinh P-U, Cheng K. Cells and cell derivatives as drug carriers for targeted delivery. Medicine in Drug Discovery. 2019;3: 100014.
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89.
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75.
Dong X, Chu D, Wang Z. Leukocyte-mediated delivery of nanotherapeutics in inflammatory and tumor sites. Theranostics. 2017;7(3):751.
Ye B, Zhao B, Wang K, Guo Y, Lu Q, Zheng L, et al. Neutrophils mediated multistage nanoparticle delivery for prompting tumor photothermal therapy. Journal of Nanobiotechnology. 2020;18(1):1–14.
Che J, Najer A, Blakney AK, McKay PF, Bellahcene M, Winter CW, et al. Neutrophils enable local and non-invasive liposome delivery to inflamed skeletal muscle and ischemic heart. Adv Mater. 2020;32(48):2003598.
Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12(7):692.
Ju C, Wen Y, Zhang L, Wang Q, Xue L, Shen J, et al. Neoadjuvant chemotherapy based on abraxane/human neutrophils cytopharmaceuticals with radiotherapy for gastric cancer. Small. 2019;15(5):1804191.
Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–41.
Zhang G, Wang L, Cui H, Wang X, Zhang G, Ma J, et al. Anti-melanoma activity of T cells redirected with a TCR-like chimeric antigen receptor. Sci Rep. 2014;4:3571.
Huang B, Abraham WD, Zheng Y, López SCB, Luo SS, Irvine DJ. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Science Transl Med. 2015;7(291):291ra94-ra94.
Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE, et al. T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale Res Lett. 2011;6(1):283.
Jones RB, Mueller S, Kumari S, Vrbanac V, Genel S, Tager AM, et al. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials. 2017;117:44–53.
Mitchell MJ, King MR. Leukocytes as carriers for targeted cancer drug delivery. Expert Opin Drug Deliv. 2015;12(3):375–92.
Pierigè F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60(2):286–95.
Yousefpour P, Chilkoti A. Co-opting biology to deliver drugs. Biotechnol Bioeng. 2014;111(9):1699–716.
Klyachko NL, Polak R, Haney MJ, Zhao Y, Neto RJG, Hill MC, et al. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials. 2017;140:79–87.
Gilboa E, Nair SK, Lyerly HK. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol Immunother. 1998;46(2):82–7.
Subklewe M, Geiger C, Lichtenegger FS, Javorovic M, Kvalheim G, Schendel DJ, et al. New generation dendritic cell vaccine for immunotherapy of acute myeloid leukemia. Cancer Immunol Immunother. 2014;63(10):1093–103.
Benencia F, Sprague L, McGinty J, Pate M, Muccioli M. Dendritic cells the tumor microenvironment and the challenges for an effective antitumor vaccination. J Biomed Biotechnol. 2012;2012.
Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, et al. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1(7):1111–34.
Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC, Barbuto JAM. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol. 2019;9(3176). https://doi.org/10.3389/fimmu.2018.03176.
Xu J, Ma Q, Zhang Y, Fei Z, Sun Y, Fan Q, et al. Yeast-derived nanoparticles remodel the immunosuppressive microenvironment in tumor and tumor-draining lymph nodes to suppress tumor growth. Nat Commun. 2022;13(1):1–15.
Wang D, Zhang B, Gao H, Ding G, Wu Q, Zhang J, et al. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC Cancer. 2014;14(1):1–7.
Sherman LS, Romagano MP, Williams SF, Rameshwar P. Mesenchymal stem cell therapies in brain disease. Semin Cell Dev Biol. 2019;95:111–9. https://doi.org/10.1016/j.semcdb.2019.03.003.
Krueger TE, Thorek DL, Denmeade SR, Isaacs JT, Brennen WN. Concise review: mesenchymal stem cell-based drug delivery: The good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;7(9):651–63.
Coccè V, Farronato D, Brini AT, Masia C, Giannì AB, Piovani G, et al. Drug loaded gingival mesenchymal stromal cells (GinPa-MSCs) inhibit in vitro proliferation of oral squamous cell carcinoma. Sci Rep. 2017;7(1):9376. https://doi.org/10.1038/s41598-017-09175-4.
Heo J-R, Hwang K-A, Kim SU, Choi K-C. A potential therapy using engineered stem cells prevented malignant melanoma in cellular and xenograft mouse models. Cancer Res Treat. 2019;51(2):797–811. https://doi.org/10.4143/crt.2018.364.
Kooreman NG, Wu JC. Tumorigenicity of pluripotent stem cells: biological insights from molecular imaging. J Royal Soc Interface. 2010;7(suppl_6):S753-S63.
Chen H-Y, Deng J, Wang Y, Wu C-Q, Li X, Dai H-W. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomaterialia. 2020.
Suryaprakash S, Lao Y-H, Cho H-Y, Li M, Ji HY, Shao D, et al. Engineered mesenchymal stem cell/nanomedicine spheroid as an active drug delivery platform for combinational glioblastoma therapy. Nano Lett. 2019;19(3):1701–5.
Tian W, Lu J, Jiao D. Stem cell membrane vesicle–coated nanoparticles for efficient tumor-targeted therapy of orthotopic breast cancer. Polym Adv Technol. 2019;30(4):1051–60.
Yang N, Ding Y, Zhang Y, Wang B, Zhao X, Cheng K, et al. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy. ACS Appl Mater Interfaces. 2018;10(27):22963–73.
Khaldoyanidi SK, Glinsky VV, Sikora L, Glinskii AB, Mossine VV, Quinn TP, et al. MDA-MB-435 human breast carcinoma cell homo-and heterotypic adhesion under flow conditions is mediated in part by Thomsen-Friedenreich antigen-galectin-3 interactions. J Biol Chem. 2003;278(6):4127–34.
Mareel M, Vleminckx K, Vermeulen S, Gao Y, Vakaet Jr L, Bracke M, Van Roy F. 3.3 Homotypic cell-cell adhesion molecules and tumor invasion. Progress in histochemistry and cytochemistry. 1992;26(1-4):95-106.
He Z, Zhang Y, Feng N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review. Mater Sci Eng, C. 2020;106: 110298.
Zhu J-Y, Zheng D-W, Zhang M-K, Yu W-Y, Qiu W-X, Hu J-J, et al. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16(9):5895–901.
Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, et al. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv Mater. 2016;28(43):9581–8.
Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, et al. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Can Res. 2003;63(13):3805–11.
Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano letters. 2014;14(4):2181–8.
Yang R, Xu J, Xu L, Sun X, Chen Q, Zhao Y, et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12(6):5121–9.
Meng X, Wang J, Zhou J, Tian Q, Qie B, Zhou G, et al. Tumor cell membrane-based peptide delivery system targeting the tumor microenvironment for cancer immunotherapy and diagnosis. Acta Biomater. 2021;127:266–75.
Cozzo AJ, Fuller AM, Makowski L. Contribution of adipose tissue to development of cancer. Compr Physiol. 2017;8(1):237.
Munteanu R, Onaciu A, Moldovan C, Zimta A-A, Gulei D, Paradiso AV, et al. Adipocyte-based cell therapy in oncology: the role of cancer-associated adipocytes and their reinterpretation as delivery platforms. Pharmaceutics. 2020;12(5):402.
Santander AM, Lopez-Ocejo O, Casas O, Agostini T, Sanchez L, Lamas-Basulto E, et al. Paracrine interactions between adipocytes and tumor cells recruit and modify macrophages to the mammary tumor microenvironment: the role of obesity and inflammation in breast adipose tissue. Cancers (Basel). 2015;7(1):143–78.
Wen D, Wang J, Van Den Driessche G, Chen Q, Zhang Y, Chen G, et al. Adipocytes as anticancer drug delivery depot. Matter. 2019;1(5):1203–14.
Zhang M, Di Martino JS, Bowman RL, Campbell NR, Baksh SC, Simon-Vermot T, et al. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov. 2018;8(8):1006–25.
Miranda F, Mannion D, Liu S, Zheng Y, Mangala LS, Redondo C, et al. Salt-inducible kinase 2 couples ovarian cancer cell metabolism with survival at the adipocyte-rich metastatic niche. Cancer Cell. 2016;30(2):273–89.
Uchibori R, Tsukahara T, Mizuguchi H, Saga Y, Urabe M, Mizukami H, et al. NF-κB activity regulates mesenchymal stem cell accumulation at tumor sites. Cancer Res. 2013;73(1):364–72.
Josiah DT, Zhu D, Dreher F, Olson J, McFadden G, Caldas H. Adipose-derived stem cells as therapeutic delivery vehicles of an oncolytic virus for glioblastoma. Mol Ther. 2010;18(2):377–85. https://doi.org/10.1038/mt.2009.265.
Choi SA, Lee JY, Kwon SE, Wang KC, Phi JH, Choi JW, et al. Human adipose tissue-derived mesenchymal stem cells target brain tumor-initiating cells. PLoS ONE. 2015;10(6): e0129292. https://doi.org/10.1371/journal.pone.0129292.
Huang W-C, Lu I-L, Chiang W-H, Lin Y-W, Tsai Y-C, Chen H-H, et al. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J Controlled Release. 2017;254:119–30.
Scioli MG, Artuso S, D’Angelo C, Porru M, D’Amico F, Bielli A, et al. Adipose-derived stem cell-mediated paclitaxel delivery inhibits breast cancer growth. PLoS ONE. 2018;13(9): e0203426.
Coccè V, Brini A, Giannì AB, Sordi V, Berenzi A, Alessandri G, et al. A nonenzymatic and automated closed-cycle process for the isolation of mesenchymal stromal cells in drug delivery applications. Stem Cells International. 2018;2018:4098140. https://doi.org/10.1155/2018/4098140.
Sun H, Burrola S, Wu J, Ding W-Q. Extracellular vesicles in the development of cancer therapeutics. Int J Mol Sci. 2020;21(17):6097.
Burgio S, Noori L, Marino Gammazza A, Campanella C, Logozzi M, Fais S, et al. Extracellular vesicles-based drug delivery systems: a new challenge and the exemplum of malignant pleural mesothelioma. Int J Mol Sci. 2020;21(15):5432.
Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189–97.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208.
Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y et al. Apoptotic cell-derived extracellular vesicles promote malignancy of glioblastoma via intercellular transfer of splicing factors. Cancer Cell. 2018;34(1):119–35. e10.
Smyth TJ, Redzic JS, Graner MW, Anchordoquy TJ. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2014;1838(11):2954–65.
Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49(3):347–60.
Kanchanapally R, Deshmukh SK, Chavva SR, Tyagi N, Srivastava SK, Patel GK, et al. Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: a comparative analysis. Int J Nanomed. 2019;14:531.
O’Loughlin AJ, Mäger I, de Jong OG, Varela MA, Schiffelers RM, El Andaloussi S, et al. Functional delivery of lipid-conjugated siRNA by extracellular vesicles. Mol Ther. 2017;25(7):1580–7.
Srivastava A, Amreddy N, Pareek V, Chinnappan M, Ahmed R, Mehta M, et al. Progress in extracellular vesicle biology and their application in cancer medicine. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2020;12(4): e1621.
Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal Biochem. 2014;448:41–9.
Cheng Z, Liu S, Wu X, Raza F, Li Y, Yuan W, et al. Autologous erythrocytes delivery of berberine hydrochloride with long-acting effect for hypolipidemia treatment. Drug Deliv. 2020;27(1):283–91.
Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell–derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Molecular Therapy-Nucleic Acids. 2013;2: e126.
Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Controlled Release. 2014;192:262–70.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–90.
Bysell H, Månsson R, Hansson P, Malmsten M. Microgels and microcapsules in peptide and protein drug delivery. Adv Drug Del Rev. 2011;63(13):1172–85.
Johnson GD, Lalancette C, Linnemann AK, Leduc F, Boissonneault G, Krawetz SA. The sperm nucleus: chromatin, RNA and the nuclear matrix. Reproduction (Cambridge, England). 2011;141(1):21.
Mattioli M, Gloria A, Mauro A, Gioia L, Barboni B. Fusion as the result of sperm–somatic cell interaction. Reproduction. 2009;138(4):679–87.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
Xu H, Sanchez MM, Magdanz V, Schwarz L, Hebenstreit F, Schmidt OG. Sperm-hybrid micromotor for drug delivery in the female reproductive tract. ar**v preprint ar**v:1703.08510. 2017.
Xu H, Medina-Sánchez M, Magdanz V, Schwarz L, Hebenstreit F, Schmidt OG. Sperm-hybrid micromotor for targeted drug delivery. ACS Nano. 2018;12(1):327–37. https://doi.org/10.1021/acsnano.7b06398.
Singh AV, Ansari MHD, Mahajan M, Srivastava S, Kashyap S, Dwivedi P, et al. Sperm cell driven microrobots—emerging opportunities and challenges for biologically inspired robotic design. Micromachines. 2020;11(4):448.
Sedighi M, Zahedi Bialvaei A, Hamblin MR, Ohadi E, Asadi A, Halajzadeh M, et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019;8(6):3167–81.
Patyar S, Joshi R, Byrav DP, Prakash A, Medhi B, Das B. Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 2010;17(1):1–9.
Kim VM, Blair AB, Lauer P, Foley K, Che X, Soares K, et al. Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies. J Immunother Cancer. 2019;7(1):1–13.
Taniguchi Si, Shimatani Y, Fujimori M. Tumor-targeting therapy using gene-engineered anaerobic-nonpathogenic Bifidobacterium longum. Bacterial Therapy Cancer. Springer. 2016:49–60.
Heap JT, Theys J, Ehsaan M, Kubiak AM, Dubois L, Paesmans K, et al. Spores of Clostridium engineered for clinical efficacy and safety cause regression and cure of tumors in vivo. Oncotarget. 2014;5(7):1761.
Gu T-W, Wang M-Z, Niu J, Chu Y, Guo K-R, Peng L-H. Outer membrane vesicles derived from E. coli as novel vehicles for transdermal and tumor targeting delivery. Nanoscale. 2020;12(36):18965–77.
Liang K, Liu Q, Li P, Luo H, Wang H, Kong Q. Genetically engineered Salmonella Typhimurium: recent advances in cancer therapy. Cancer Lett. 2019;448:168–81.
Van Mellaert L, Barbé S, Anné J. Clostridium spores as anti-tumour agents. Trends Microbiol. 2006;14(4):190–6.
Duong MT-Q, Qin Y, You S-H, Min J-J. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 2019;51(12):1–15.
Shi L, Sheng J, Wang M, Luo H, Zhu J, Zhang B, et al. Combination therapy of TGF-β blockade and commensal-derived probiotics provides enhanced antitumor immune response and tumor suppression. Theranostics. 2019;9(14):4115.
Ganai S, Arenas RB, Sauer JP, Bentley B, Forbes NS. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther. 2011;18(7):457–66.
Lee C, Lin S, Liu J, Chang W, Hsieh J, Wang W. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther. 2014;21(3):309–16.
Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69(14):5860–6.
Hosseinidoust Z, Mostaghaci B, Yasa O, Park B-W, Singh AV, Sitti M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev. 2016;106:27–44.
Li M, Zhou H, Yang C, Wu Y, Zhou X, Liu H, et al. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J Control Release. 2020;323:253–68.
Huang W, Shu C, Hua L, Zhao Y, **e H, Qi J, et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020;108:300–12.
Liu X, Jiang S, Piao L, Yuan F. Radiotherapy combined with an engineered of Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp Anim. 2016:16–0033.
Dresselaers T, Theys J, Nuyts S, Wouters B, de Bruijn E, Anné J, et al. Non-invasive 19 F MR spectroscopy of 5-fluorocytosine to 5-fluorouracil conversion by recombinant Salmonella in tumours. Br J Cancer. 2003;89(9):1796–801.
Nemunaitis J, Cunningham C, Senzer N, Kuhn J, Cramm J, Litz C et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10(10):737–44.
Zou S, Wang B, Wang C, Wang Q, Zhang L. Cell membrane-coated nanoparticles: research advances. Nanomedicine. 2020;15(06):625–41.
Li J, Wang X, Zheng D, Lin X, Wei Z, Zhang D, et al. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomaterials science. 2018;6(7):1834–45.
Rao L, Cai B, Bu L-L, Liao Q-Q, Guo S-S, Zhao X-Z, et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11(4):3496–505.
Rao L, Bu LL, Cai B, Xu JH, Li A, Zhang WF, et al. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater. 2016;28(18):3460–6.
Ren X, Zheng R, Fang X, Wang X, Zhang X, Yang W, et al. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials. 2016;92:13–24.
Zhang X, He S, Ding B, Qu C, Zhang Q, Chen H, et al. Cancer cell membrane-coated rare earth doped nanoparticles for tumor surgery navigation in NIR-II imaging window. Chem Eng J. 2020;385: 123959.
Wang C, Wu B, Wu Y, Song X, Zhang S, Liu Z. Camouflaging nanoparticles with brain metastatic tumor cell membranes: a new strategy to traverse blood–brain barrier for imaging and therapy of brain tumors. Adv Funct Mater. 2020;30(14):1909369.
Li S, Jiang W, Yuan Y, Sui M, Yang Y, Huang L, et al. Delicately designed cancer cell membrane-camouflaged nanoparticles for targeted 19F MR/PA/FL imaging-guided photothermal therapy. ACS Appl Mater Interfaces. 2020;12(51):57290–301.
Lu H, Guo K, Cao Y, Yang F, Wang D, Dou L, et al. Cancer cell membrane vesicle for multiplex MicroRNA imaging in living cells. Anal Chem. 2019;92(2):1850–5.
Chen Q, Zhang L, Li L, Tan M, Liu W, Liu S, et al. Cancer cell membrane-coated nanoparticles for bimodal imaging-guided photothermal therapy and docetaxel-enhanced immunotherapy against cancer. Journal of nanobiotechnology. 2021;19(1):1–22.
Li J, Zhen X, Lyu Y, Jiang Y, Huang J, Pu K. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano. 2018;12(8):8520–30.
Zhang K, Cao Y, Kuang Y, Liu M, Chen Y, Wang Z, et al. Gd 2 O 3 and GH combined with red blood cells to improve the sensitivity of contrast agents for cancer targeting MR imaging. Biomaterials science. 2017;5(1):46–9.
Choi J, Kim H-Y, Ju EJ, Jung J, Park J, Chung H-K, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33(16):4195–203.
Huang X, Zhang F, Wang H, Niu G, Choi KY, Swierczewska M, et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials. 2013;34(7):1772–80.
Ahn S, Jung SY, Seo E, Lee SJ. Gold nanoparticle-incorporated human red blood cells (RBCs) for X-ray dynamic imaging. Biomaterials. 2011;32(29):7191–9.
Kravtzoff R, Ropars C, Desbois I, Chassaigne M, Lamagnere J, Colombat P, et al. Improved pharmacodynamics of L-asparaginase-loaded in human red blood cells. Eur J Clin Pharmacol. 1996;49(6):465–70.
Li C-X, Qi Y-D, Feng J, Zhang X-Z. Cell-based bio-hybrid delivery system for disease treatments. Advanced Nanobiomed Research. 2021;1(10):2000052.
Wu S-H, Hsieh C-C, Hsu S-C, Yao M, Hsiao J-K, Wang S-W et al. RBC-derived vesicles as a systemic delivery system of doxorubicin for lysosomal-mitochondrial axis-improved cancer therapy. J Adv Res. 2020.
Zhang D, Zhou C, Liu F, Ding T, Wang Z. Red blood cells membrane vehicle co-delivering DOX and IR780 for effective prostate cancer therapy. J Mater Res. 2020;35(22):3116–23.
Harisa GI, Ibrahim MF, Alanazi F, Shazly GA. Engineering erythrocytes as a novel carrier for the targeted delivery of the anticancer drug paclitaxel. Saudi Pharmaceutical Journal. 2014;22(3):223–30. https://doi.org/10.1016/j.jsps.2013.06.007.
Wang GP, Guan YS, ** XR, Jiang SS, Lu ZJ, Wu Y, et al. Development of novel 5-fluorouracil carrier erythrocyte with pharmacokinetics and potent antitumor activity in mice bearing malignant ascites. J Gastroenterol Hepatol. 2010;25(5):985–90. https://doi.org/10.1111/j.1440-1746.2009.06155.x.
Jiang Q, Liu Y, Guo R, Yao X, Sung S, Pang Z, et al. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308.
Fu Q, Lv P, Chen Z, Ni D, Zhang L, Yue H, et al. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane. Nanoscale. 2015;7(9):4020–30.
Su J, Sun H, Meng Q, Yin Q, Zhang P, Zhang Z, et al. Bioinspired nanoparticles with NIR-controlled drug release for synergetic chemophotothermal therapy of metastatic breast cancer. Adv Func Mater. 2016;26(41):7495–506.
Zhang X, **ong J, Wang K, Yu H, Sun B, Ye H, et al. Erythrocyte membrane-camouflaged carrier-free nanoassembly of FRET photosensitizer pairs with high therapeutic efficiency and high security for programmed cancer synergistic phototherapy. Bioactive Materials. 2021;6(8):2291–302.
Daniyal M, Jian Y, **ao F, Sheng W, Fan J, **ao C, et al. Development of a nanodrug-delivery system camouflaged by erythrocyte membranes for the chemo/phototherapy of cancer. Nanomedicine. 2020;15(07):691–709.
Lin Y, Zhong Y, Chen Y, Li L, Chen G, Zhang J, et al. Ligand-modified erythrocyte membrane-cloaked metal–organic framework nanoparticles for targeted antitumor therapy. Mol Pharm. 2020;17(9):3328–41.
Zhai Z, Xu P, Yao J, Li R, Gong L, Yin Y, et al. Erythrocyte-mimicking paclitaxel nanoparticles for improving biodistributions of hydrophobic drugs to enhance antitumor efficacy. Drug Delivery. 2020;27(1):387–99.
Wang Y, Luan Z, Zhao C, Bai C, Yang K. Target delivery selective CSF-1R inhibitor to tumor-associated macrophages via erythrocyte-cancer cell hybrid membrane camouflaged pH-responsive copolymer micelle for cancer immunotherapy. Eur J Pharm Sci. 2020;142: 105136.
Lian Y, Wang X, Guo P, Li Y, Raza F, Su J, et al. Erythrocyte membrane-coated arsenic trioxide-loaded sodium alginate nanoparticles for tumor therapy. Pharmaceutics. 2020;12(1):21.
Chen Z, Wang W, Li Y, Wei C, Zhong P, He D, et al. Folic acid-modified erythrocyte membrane loading dual drug for targeted and chemo-photothermal synergistic cancer therapy. Mol Pharm. 2020.
Chen S, Ren Y, Duan P. Biomimetic nanoparticle loading obatoclax mesylate for the treatment of non-small-cell lung cancer (NSCLC) through suppressing Bcl-2 signaling. Biomed Pharmacother. 2020;129: 110371.
Zheng D, Yu P, Wei Z, Zhong C, Wu M, Liu X. RBC Membrane camouflaged semiconducting polymer nanoparticles for near-infrared photoacoustic imaging and photothermal therapy. Nano-Micro Letters. 2020;12(1):1–17.
Huang X, Shang W, Deng H, Zhou Y, Cao F, Fang C, et al. Clothing spiny nanoprobes against the mononuclear phagocyte system clearance in vivo: photoacoustic diagnosis and photothermal treatment of early stage liver cancer with erythrocyte membrane-camouflaged gold nanostars. Appl Mater Today. 2020;18: 100484.
Bidkar AP, Sanpui P, Ghosh SS. Red blood cell-membrane-coated poly (lactic-co-glycolic acid) nanoparticles for enhanced chemo-and hypoxia-activated therapy. ACS Appl Bio Mater. 2019;2(9):4077–86.
Sarkar S, Alam MA, Shaw J, Dasgupta AK. Drug delivery using platelet cancer cell interaction. Pharm Res. 2013;30(11):2785–94. https://doi.org/10.1007/s11095-013-1097-1.
Pan V, Siva PN, Modery-Pawlowski CL, Singh Sekhon UD, Gupta AS. Targeted killing of metastatic cells using a platelet-inspired drug delivery system. RSC Adv. 2015;5(57):46218–28. https://doi.org/10.1039/C5RA05339K.
Xu P, Zuo H, Chen B, Wang R, Ahmed A, Hu Y, et al. Doxorubicin-loaded platelets as a smart drug delivery system: an improved therapy for lymphoma. Sci Rep. 2017;7(1):42632. https://doi.org/10.1038/srep42632.
Wang H, Bremner DH, Wu K, Gong X, Fan Q, **e X, et al. Platelet membrane biomimetic bufalin-loaded hollow MnO2 nanoparticles for MRI-guided chemo-chemodynamic combined therapy of cancer. Chem Eng J. 2020;382: 122848. https://doi.org/10.1016/j.cej.2019.122848.
Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nature Biomedical Engineering. 2017;1(2):0011. https://doi.org/10.1038/s41551-016-0011.
Wu Y-W, Huang C-C, Changou CA, Lu L-S, Goubran H, Burnouf T. Clinical-grade cryopreserved doxorubicin-loaded platelets: role of cancer cells and platelet extracellular vesicles activation loop. J Biomed Sci. 2020;27(1):45. https://doi.org/10.1186/s12929-020-00633-2.
Kim MW, Lee G, Niidome T, Komohara Y, Lee R, Park YI. Platelet-like gold nanostars for cancer therapy: the ability to treat cancer and evade immune reactions. Front Bioeng Biotechnol. 2020;8:133. https://doi.org/10.3389/fbioe.2020.00133.
Xu L, Su T, Xu X, Zhu L, Shi L. Platelets membrane camouflaged irinotecan-loaded gelatin nanogels for in vivo colorectal carcinoma therapy. Journal of Drug Delivery Science and Technology. 2019;53: 101190. https://doi.org/10.1016/j.jddst.2019.101190.
Wang H, Wu J, Williams GR, Fan Q, Niu S, Wu J, et al. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J Nanobiotechnology. 2019;17(1):60. https://doi.org/10.1186/s12951-019-0494-y.
Bang KH, Na YG, Huh HW, Hwang SJ, Kim MS, Kim M et al. The delivery strategy of paclitaxel nanostructured lipid carrier coated with platelet membrane. Cancers (Basel). 2019;11(6). https://doi.org/10.3390/cancers11060807.
Ding K, Zheng C, Sun L, Liu X, Yin Y, Wang L. NIR light-induced tumor phototherapy using ICG delivery system based on platelet-membrane-camouflaged hollow bismuth selenide nanoparticles. Chin Chem Lett. 2019. https://doi.org/10.1016/j.cclet.2019.10.040.
Wang H, Wu J, Williams GR, Fan Q, Niu S, Wu J, et al. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. Journal of Nanobiotechnology. 2019;17(1):60. https://doi.org/10.1186/s12951-019-0494-y.
Xu P, Zuo H, Zhou R, Wang F, Liu X, Ouyang J, et al. Doxorubicin-loaded platelets conjugated with anti-CD22 mAbs: a novel targeted delivery system for lymphoma treatment with cardiopulmonary avoidance. Oncotarget. 2017;8(35):58322–37. https://doi.org/10.18632/oncotarget.16871.
Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater (Deerfield Beach, Fla.). 2015;27(44):7043–50. https://doi.org/10.1002/adma.201503323.
Díaz A, Saxena V, González J, David A, Casañas B, Carpenter C, et al. Zirconium phosphate nano-platelets: a novel platform for drug delivery in cancer therapy. Chem Commun. 2012;48(12):1754–6. https://doi.org/10.1039/C2CC16218K.
Bu LL, Rao L, Yu GT, Chen L, Deng WW, Liu JF, et al. Cancer stem cell-platelet hybrid membrane-coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma. Adv Func Mater. 2019;29(10):1807733.
Yoon A-R, Hong J, Li Y, Shin HC, Lee H, Kim HS, et al. Mesenchymal stem cell–mediated delivery of an oncolytic adenovirus enhances antitumor efficacy in hepatocellular carcinoma. Can Res. 2019;79(17):4503–14.
Salehi H, Al-Arag S, Middendorp E, Gergely C, Cuisinier F, Orti V. Dental pulp stem cells used to deliver the anticancer drug paclitaxel. Stem Cell Res Ther. 2018;9(1):1–10.
Takayama Y, Kusamori K, Tsukimori C, Shimizu Y, Hayashi M, Kiyama I, et al. Anticancer drug-loaded mesenchymal stem cells for targeted cancer therapy. J Control Release. 2021;329:1090–101.
Gao C, Lin Z, Jurado-Sánchez B, Lin X, Wu Z, He Q. Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small. 2016;12(30):4056–62.
Zhang J, Miao Y, Ni W, **ao H, Zhang J. Cancer cell membrane coated silica nanoparticles loaded with ICG for tumour specific photothermal therapy of osteosarcoma. Artificial cells, nanomedicine, and biotechnology. 2019;47(1):2298–305.
Lai P-Y, Huang R-Y, Lin S-Y, Lin Y-H, Chang C-W. Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv. 2015;5(119):98222–30.
Mooney R, Weng Y, Tirughana-Sambandan R, Valenzuela V, Aramburo S, Garcia E, et al. Neural stem cells improve intracranial nanoparticle retention and tumor-selective distribution. Future Oncol. 2014;10(3):401–15.
Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, et al. Silica nanorattle–doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano. 2011;5(9):7462–70.
Liu Y, Zhao J, Jiang J, Chen F, Fang X. Doxorubicin delivered using nanoparticles camouflaged with mesenchymal stem cell membranes to treat colon cancer. Int J Nanomed. 2020;15:2873.
Feng J, Wang S, Wang Y, Wang L. Stem cell membrane–camouflaged bioinspired nanoparticles for targeted photodynamic therapy of lung cancer. J Nanopart Res. 2020;22(7):1–11.
De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, et al. Tumor-targeted interferon-α delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14(4):299–311. https://doi.org/10.1016/j.ccr.2008.09.004.
Lang T, Dong X, Huang Y, Ran W, Yin Q, Zhang P, et al. Ly6Chi Monocytes delivering pH-sensitive micelle loading paclitaxel improve targeting therapy of metastatic breast cancer. Adv Func Mater. 2017;27(26):1701093. https://doi.org/10.1002/adfm.201701093.
Cao X, Hu Y, Luo S, Wang Y, Gong T, Sun X, et al. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharmaceutica Sinica B. 2019;9(3):575–89. https://doi.org/10.1016/j.apsb.2018.12.009.
Liu L, Chen Q, Ruan C, Chen X, He X, Zhang Y, et al. Nano-engineered lymphocytes for alleviating suppressive tumor immune microenvironment. Appl Mater Today. 2019;16:273–9. https://doi.org/10.1016/j.apmt.2019.06.009.
Luo X, Hu L, Zheng H, Liu M, Liu X, Li C, et al. Neutrophil-mediated delivery of pixantrone-loaded liposomes decorated with poly(sialic acid)–octadecylamine conjugate for lung cancer treatment. Drug Delivery. 2018;25(1):1200–12. https://doi.org/10.1080/10717544.2018.1474973.
Kang T, Zhu Q, Wei D, Feng J, Yao J, Jiang T, et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11(2):1397–411.
Thomsen T, Ayoub AB, Psaltis D, Klok H-A. Fluorescence-based and fluorescent label-free characterization of polymer nanoparticle decorated T cells. Biomacromolecules. 2020.
Qiu Y, Ren K, Zhao W, Yu Q, Guo R, He J, et al. A “dual-guide” bioinspired drug delivery strategy of a macrophage-based carrier against postoperative triple-negative breast cancer recurrence. J Control Release. 2021;329:191–204.
Hou T, Wang T, Mu W, Yang R, Liang S, Zhang Z, et al. Nanoparticle-loaded polarized-macrophages for enhanced tumor targeting and cell-chemotherapy. Nano-Micro Letters. 2021;13(1):1–20.
Xu M, Chen Q, Li J, Peng L, Ding L. Dendritic cell-derived exosome-entrapped fluorouracil can enhance its anti-colon cancer effect. J BUON: official journal of the Balkan Union of Oncology. 2020;25(3):1413-22.
Chowdhury S, Castro S, Coker C, Hinchliffe TE, Arpaia N, Danino T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. 2019;25(7):1057–63.
Han J-W, Choi YJ, Cho S, Zheng S, Ko SY, Park J-O, et al. Active tumor-therapeutic liposomal bacteriobot combining a drug (paclitaxel)-encapsulated liposome with targeting bacteria (Salmonella Typhimurium). Sens Actuators B Chem. 2016;224:217–24.
Quispe-Tintaya W, Chandra D, Jahangir A, Harris M, Casadevall A, Dadachova E, et al. Nontoxic radioactive Listeriaat is a highly effective therapy against metastatic pancreatic cancer. Proc Natl Acad Sci. 2013;110(21):8668–73.
Chen Q, Huang G, Wu W, Wang J, Hu J, Mao J, et al. A hybrid eukaryotic–prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv Mater. 2020;32(16):1908185.
MacDiarmid JA, Langova V, Bailey D, Pattison ST, Pattison SL, Christensen N, et al. Targeted doxorubicin delivery to brain tumors via minicells: proof of principle using dogs with spontaneously occurring tumors as a model. PLoS ONE. 2016;11(4): e0151832.
Zhang Y, Ji W, He L, Chen Y, Ding X, Sun Y et al. E. coli Nissle 1917-derived minicells for targeted delivery of chemotherapeutic drug to hypoxic regions for cancer therapy. Theranostics. 2018;8(6):1690.
Acknowledgements
The authors would like to acknowledge Shiraz University of Medical Sciences for financially supporting the research. They would also like to appreciate Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for her invaluable assistance in editing the manuscript.
Funding
This work was funded by Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
All the authors significantly contributed to the study, as follows (author-wise): Vahid Alimardani, investigation, conceptualization, writing, and original draft preparation; Zahra Rahiminezhad, Mahvash Dehghan Khold, Ghazal Farahavar, Mahboobeh Jafari, Mehdi Abedi, Leila Moradi, and Uranous Niroumand, writing and original draft preparation; Mohammad Ashfaq, Samirasadat Abolmaali, and Gholamhossein Yousefi, conceptualization and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All authors have read and approved the final manuscript.
Consent for publication
On behalf of the authors, the corresponding author hereby ensures the consent of all authors for submitting the article to Drug Delivery and Translational Research Journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alimardani, V., Rahiminezhad, Z., DehghanKhold, M. et al. Nanotechnology-based cell-mediated delivery systems for cancer therapy and diagnosis. Drug Deliv. and Transl. Res. 13, 189–221 (2023). https://doi.org/10.1007/s13346-022-01211-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01211-9