Abstract
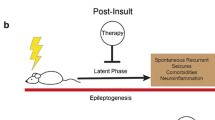
A major unmet medical need is the lack of treatments to prevent (or modify) epilepsy in patients at risk, for example, after epileptogenic brain insults such as traumatic brain injury, stroke, or prolonged acute symptomatic seizures like complex febrile seizures or status epilepticus. Typically, following such brain insults there is a seizure-free interval (“latent period”), lasting months to years before the onset of spontaneous recurrent epileptic seizures. The latent period after a brain insult offers a window of opportunity in which an appropriate treatment may prevent or modify the epileptogenic process induced by a brain insult. A similar latent period occurs in patients with epileptogenic gene mutations. Studies using animal models of epilepsy have led to a greater understanding of the factors underlying epileptogenesis and have provided significant insight into potential targets by which the development of epilepsy may be prevented or modified. This review focuses largely on some of the most common animal models of epileptogenesis and their potential utility for evaluating proposed antiepileptogenic therapies and identifying useful biomarkers. The authors also describe some of the limitations of using animal models in the search for therapies that move beyond the symptomatic treatment of epilepsy. Promising results of previous studies designed to evaluate antiepileptogenesis and the role of monotherapy versus polytherapy approaches are also discussed. Recent data from both models of genetic and acquired epilepsies strongly indicate that it is possible to prevent or modify epileptogenesis, and, hopefully, such promising results can ultimately be translated into the clinic.


Similar content being viewed by others
References
Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov 2010;9:68-82.
Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 2011;52:657-678.
Löscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for antiepileptic drug discovery and development. A joint endeavor of academia and industry. Nat Rev Drug Discov 2013;12:757-776.
Löscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev 2010;62:668-700.
Pitkänen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol 2011;10:173-186.
White HS. Animal models for evaluating antiepileptogenesis. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies. 4th edition. Oxford University Press, New York, 2012, pp. 1041-1054.
Galanopoulou AS, Simonato M, French JA, O’Brien TJ. Joint AES/ILAE translational workshop to optimize preclinical epilepsy research. Epilepsia 2013;54(Suppl. 4):1-2.
Coenen AM, Van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet 2003;33:635-655.
Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 2005;131:759-768.
Baraban SC, Dinday MT, Hortopan GA. Drug screening and transcriptomic analysis in Scn1a zebrafish mutants identifies potential lead compound for Dravet Syndrome. Nature Comm 2013;4:2410.
Baraban SC, Löscher W. New approaches to model epilepsy and identify promising drug treatments. In: Scharfman H, Buckmaster PS (eds) Issues in clinical epileptology. A view from the bench. Springer, Heidelberg, 2013.
Pitkänen A, Nehlig A, Brooks-Kayal AR, Dudek FE, Friedman D, Galanopoulou AS, et al. Issues related to development of antiepileptogenic therapies. Epilepsia 2013;54(Suppl. 4):35-43.
Löscher W. The pharmacokinetics of antiepileptic drugs in rats: consequences for maintaining effective drug levels during prolonged drug administration in rat models of epilepsy. Epilepsia 2007;48:1245-1258.
Simmonds MA, Turner JP. Potentiators of responses to activation of gamma-aminobutyric acid (GABAA) receptors. Neuropharmacology 1987;26:923-930.
Löscher W, Nolting B, Fassbender CP. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. I. The influence of administration vehicles. Epilepsy Res 1990;7:173-181.
Costa E, Guidotti A. Benzodiazepines on trial: A research strategy for their rehabilitation. Trends Pharmacol Sci 1996;17:192-200.
Voss J, Sanchez C, Michelsen S, Ebert B. Rotarod studies in the rat of the GABAA receptor agonist gaboxadol: lack of ethanol potentiation and benzodiazepine cross-tolerance. Eur J Pharmacol 2003;482:215-222.
Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 2009;29:6964-6972.
Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci 2009;29:8259-8269.
Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol 2008;63:444-453.
Heng K, Haney MM, Buckmaster PS. High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia 2013;54:1535-1541.
Engel J, Jr., Pitkänen A, Loeb JA, Dudek FE, Bertram EH, III, Cole AJ, et al. Epilepsy biomarkers. Epilepsia 2013;54(Suppl. 4):61-69.
Sloviter RS. Hippocampal epileptogenesis in animal models of mesial temporal lobe epilepsy with hippocampal sclerosis: the importance of the “latent period” and other concepts. Epilepsia 2008;49(Suppl. 9):85-92.
Sloviter RS, Bumanglag AV. Defining “epileptogenesis” and identifying “antiepileptogenic targets” in animal models of acquired temporal lobe epilepsy is not as simple as it might seem. Neuropharmacology 2013;69:3-15.
Löscher W, Schmidt D. Epilepsy: Perampanel-new promise for refractory epilepsy? Nat Rev Neurol 2012;8:661-662.
Brandt C, Gastens AM, Sun MZ, Hausknecht M, Löscher W. Treatment with valproate after status epilepticus: Effect on neuronal damage, epileptogenesis, and behavioral alterations in rats. Neuropharmacology 2006;51:789-804.
Langer M, Brandt C, Zellinger C, Löscher W. Therapeutic window of opportunity for the neuroprotective effect of valproate versus the competitive AMPA receptor antagonist NS1209 following status epilepticus in rats. Neuropharmacology 2011;61:1033-1047.
Bumanglag AV, Sloviter RS. Minimal latency to hippocampal epileptogenesis and clinical epilepsy after perforant pathway stimulation-induced status epilepticus in awake rats. J Comp Neurol 2008;510:561-580.
Armstrong C, Morgan RJ, Soltesz I. Pursuing paradoxical proconvulsant prophylaxis for epileptogenesis. Epilepsia 2009;50:1657-1669.
Sloviter RS. Progress on the issue of excitotoxic injury modification vs. real neuroprotection; implications for post-traumatic epilepsy. Neuropharmacology 2011;61:1048-1050.
Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS et al. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia 2008;49:400-409.
Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, et al. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia 2010;51:1560-1569.
Russo E, Citraro R, Scicchitano F, De Fazio S, Perrotta I, Di Paola ED, et al. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia 2011;52:1341-1350.
Dezsi G, Ozturk E, Stanic D, Powell KL, Blumenfeld H, O’Brien TJ, et al. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia 2013;54:635-643.
Silver JM, Shin C, McNamara JO. Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilepsy. Ann Neurol 1991;29:356-363.
Löscher W, Hönack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther 1998;284:474-479.
Löscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res 2002;50:105-123.
Eastman CL, Verley DR, Fender JS, Stewart TH, Nov E, Curia G, et al. Antiepileptic and antiepileptogenic performance of carisbamate after head injury in the rat: blind and randomized studies. J Pharmacol Exp Ther 2011;336:779-790.
D’Ambrosio R, Eastman CL, Darvas F, Fender JS, Verley DR, Farin FM, et al. Mild passive focal cooling prevents epileptic seizures after head injury in rats. Ann Neurol 2013;73:199-209.
Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia 2009;50(Suppl. 2):10-13.
Dichter MA. Posttraumatic epilepsy: the challenge of translating discoveries in the laboratory to pathways to a cure. Epilepsia 2009;50(Suppl. 2):41-45.
Furlan AJ. Challenges in acute ischemic stroke clinical trials. Curr Cardiol Rep 2012;14:761-766.
Mani R, Pollard J, Dichter MA. Human clinical trails in antiepileptogenesis. Neurosci Lett 2011;497:251-256.
Liu G, Gu B, He XP, Joshi RB, Wackerle HD, Rodriguiz RM, et al. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron 2013;79:31-38.
Bertram EH, Zhang DX, Mangan P, Fountain N, Rempe D. Functional anatomy of limbic epilepsy: a proposal for central synchronization of a diffusely hyperexcitable network. Epilepsy Res 1998;32:194-205.
Engel J, Jr, Thompson PM, Stern JM, Staba RJ, Bragin A, et al. Connectomics and epilepsy. Curr Opin Neurol 2013;26:186-194.
Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol 2012;8:669-677.
Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 2008;4:682-690.
Ainsworth C. Networking for new drugs. Nat Med 2011;17:1166-1168.
Loeb JA. Identifying targets for preventing epilepsy using systems biology. Neurosci Lett 2011;497:205-212.
Margineanu DG. Systems biology impact on antiepileptic drug discovery. Epilepsy Res 2012;98:104-115.
Vezzani A. Before epilepsy unfolds: finding the epileptogenesis switch. Nat Med 2012;18:1626-1627.
Mazzuferi M, Kumar G, van Eyll J, Danis B, Foerch P, Kaminski RM. Nrf2 defense pathway: Experimental evidence for its protective role in epilepsy. Ann Neurol 2013;74:560-568.
Löscher W, Rundfeldt C, Hönack D. Low doses of NMDA receptor antagonists synergistically increase the anticonvulsant effect of the AMPA receptor antagonist NBQX in the kindling model of epilepsy. Eur J Neurosci 1993;5:1545-1550.
Löscher W, Hönack D. Over-additive anticonvulsant effect of memantine and NBQX in kindled rats. Eur J Pharmacol 1994;259:R3-R5.
Rogawski MA, Donevan SD. AMPA receptors in epilepsy and as targets for antiepileptic drugs. Adv Neurol 1999;79:947-963.
Löscher W, Puskarjov M, Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology 2013;69:62-74.
Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, et al. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol 2008;4:490-503.
Miles R, Blaesse P, Huberfeld G, Wittner L, Kaila K. Chloride homeostasis and GABA signaling in temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) jasper’s basic mechanisms of the epilepsies. 4th edition. Oxford University Press, New York, 2012, pp. 581-590.
Brandt C, Nozadze M, Heuchert N, Rattka M, Löscher W. Disease-modifying effects of phenobarbital and the NKCC1 inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. J Neurosci 2010;30:8602-8612.
Kahle KT, Staley KJ. The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures. Neurosurg Focus 2008;25:1-8.
Koyama R, Tao K, Sasaki T, Ichikawa J, Miyamoto D, Muramatsu R, et al. GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nat Med 2012;18:1271-1278.
Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology 2013;69:16-24.
Kwon YS, Pineda E, Auvin S, Shin D, Mazarati A, Sankar R. Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J Neuroinflammation 2013;10:30.
McClelland S, Flynn C, Dube C, Richichi C, Zha Q, Ghestem A, et al. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol 2011;70:454-464.
Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G, et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med 2012;18:1087-1094.
Pitkänen A, Narkilahti S, Bezvenyuk Z, Haapalinna A, Nissinen J. Atipamezole, an alpha(2)-adrenoceptor antagonist, has disease modifying effects on epileptogenesis in rats. Epilepsy Res 2004;61:119-140.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
White, H.S., Löscher, W. Searching for the Ideal Antiepileptogenic Agent in Experimental Models: Single Treatment Versus Combinatorial Treatment Strategies. Neurotherapeutics 11, 373–384 (2014). https://doi.org/10.1007/s13311-013-0250-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-013-0250-1