Abstract
The aim of this research was to determine the time of blood coagulation of in vivo cuts that treated by the non-thermal atmospheric pressure plasma jet. Also, the effect of different treatment times on wound healing has been studied. The non-thermal atmospheric pressure plasma jet working in helium gas has been used. The averaged treatment time of 8.6 s for in vivo cuts on the Balb/c mouse liver showed the complete blood coagulation. Also, the effect of tretament time on wound healing has been studied by applying plasma on the wounds for different times (10, 20, 30, 40 and 50 s). It was obtained from morphological analysis that the treatment groups of 30 s, 40 s and 50 s cause the wounds to be healed faster than the groups 10 s and 20 s.The histological analysis showed that in 30 s and 40 s treatment time groups, the repair process of treated wounds has been accelerated, while for 50 s group, it has not been completed, yet. The 30 s treatment time has been chosen because of imposing lower dose to living tissue. The treated wound area reduction ratios against the control wound reduction ratios for 30 s group were obtained 78%, 77% and 63% at the 3rd, 5th and 8th days, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, new researches have been done on the therapeutic application using non-thermal atmospheric pressure plasma [1,2,3]. Recently, it has been found that the non-thermal atmospheric pressure plasma can be applied to living tissues [4], inducing blood coagulation and killing bacteria on the wounds without significant side effect or heating [5]. Non-thermal plasma is known to catalyze biochemical activities when applied on tissue and is able to regulate cellular processes such as proliferation, differentiation, and apoptosis [6].
Reactive nitrogen species (RNS) and reactive oxygen species (ROS) exist in non-thermal atmospheric pressure plasma [7,8,9]. The important parameters in medical plasma are the electron and different ion densities, electron temperature, UV irradiation, different radical densities and etc [10]. The active, charged particles (electrons, positive and negative ions) and uncharged atoms and molecules (such as O3, OH, H2O2, NO, OH radicals etc.) are the important species in therapeutic and medical applications [11, 12]. In other words, the reactive oxygen and nitrogen species (RONS) have the key role in medical applications [13]. The “controllable plasma” has capability to provide RONS with possible positive affect.In the inflammatory phase, the important phase of wound healing, the bacterial infection of wound may happen and the inflammatory phase may prevent the wound healing process from entering the proliferative phase and can even cause damage to the extracellular matrix (ECM) [14]. Fortunately, the ROS and RNS produced by atmospheric pressure cold plasma can inactivate bacteria and improve the blood oxygenation level in tissue [15].
Some studies have been done on the effect of non-thermal atmospheric pressure plasma on blood coagulation and wound healing process. The effects of floating electrode dielectric barrier discharge plasma in air on the blood coagulation and living tissue sterilization were investigated by Fridman et al. [4]. They found that 15 s treatment of the in vitro blood drop, leads to complete coagulation in 1 min. Also, they showed that the treatment of human spleen (in vivo application) for 30 s results in blood coagulation with the cut temperature remained at room temperature. Janani et al. [16] investigated on the effect of the cold argon plasma device on decreasing the coagulation time in vitro and in vivo. They showed that the blood drops on the slide coagulated completely after plasma exposure treatment, whereas the coagulation took 10 min without plasma treatment. Also, they found that 12 s treatment of incised liver of rats resulted in complete blood coagulation while it lasted 4.5 min without treatment. Sakakita and coworkers [17] investigated on the red blood cell coagulation induced by low-temperature plasma treatment. They authenticated that a different clot formation mechanism involving hemolysis of erythrocytes to coagulate from the already known clot formation process linked with plasma treatment. Xu et al. [18] studied the effect of an atmospheric pressure plasma jet generated in argon gas on the skin wound healing of the Balb/c mice. They compared natural wound healing with stimulated wound healing treated daily with argon plasma jet for different lengths of time on 14 consecutive days. They found that 30 s treatment time had better effect on the wound healing than the other treatment time groups. The effectiveness of the kINPen atmospheric plasma source for wound healing by applying different treatment times to the wounds of rats was investigated by Dowling et al. [19]. They concluded that the 30 s treatment performed best among the other treatment time groups. Garcia-Alcantara et al. [20] worked on the accelerating acute wound healing on the mice skin in vivo by combining treatment of argon and helium plasma needle. It was concluded from their research that after three treatments with argon plasma, the blood produced in the wound was coagulated. Salehi et al. [21] worked on the effect of atmospheric-pressure plasma on process of wound healing. They used atmospheric pressure plasma jet for wound treatment. The effect of the atmospheric pressure plasma on wound healing in diabetic rats has been studied by Fathollah et al. [22]. Shahbazi Rad and Abbasi Davani [23, 24] constructed a non-thermal atmospheric pressure dielectric barrier discharge plasma source and studied the effect of grid on wound healing application. Nakajima et al. [25] worked on the effect of cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. They found that plasma may promote the late phase of inflammation, accelerate re-epithelialization and increase wound contraction. Nastuta et al. [26] studied the stimulation of wound healing by helium atmospheric pressure plasma treatment. They reported that the dynamics of the skin regeneration process was modified: the epidermis re-epithelization was accelerated, while the recovery of superficial dermis was slowed down.
In this study, a non-thermal atmospheric pressure plasma jet which works in helium gas was constructed. The homemade sinusoidal power supply with intermediate frequency has been used to drive discharges. The purpose of this article was determining the appropriate treatment time for in vivo blood coagulation and wound healing of mice skin. The constructed helium plasma jet has been applied to the in vivo cuts on the livers of the mice to obtain the required treatment time for blood coagulation. Also, the effect of helium plasma jet on the wound healing acceleration has been investigated by applying plasma jet at different treatment times to the induced skin wounds of Balb/c mice. The morphological parameters of wounds treated with different treatment times and control wounds have been measured and compared. The voltage and current waveforms and the optical emission spectrum of the helium plasma jet have been measured in this work. Also, the histological microscopy images of control and treated samples have been obtained.
Materials and methods
Plasma setup and diagnostics
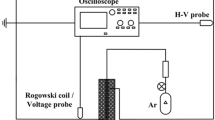
An atmospheric pressure plasma jet working in helium gas has been used in this research. The homemade helium plasma jet is shown in Fig. 1a, b shows the cross-sectional schematic of plasma jet.
The helium plasma jet has been constructed using quartz tube with the inner and outer diameter of 4 mm and 6 mm and with the height of 80 mm. This device includes two electrodes; one as an outer Aluminium electrode placed at 10 mm from the tube nozzle and another as 1 mm diameter grounded needle which is located at the tube center. The constructed plasma jet works with the homemade sinusoidal power supply. The power supply produces sine waves with the output values up to 30 kVp−p and the frequency range between 10 and 12 kHz. The high voltage is applied to the outer electrode, while the centered needle is grounded. The plasma jet has been allowed to work with two gases (Argon and Helium), either individually. In this research, the 99.999% pure helium gas with the flow rate between 0.5 and 2 slm was used as working gas. The discharge plasma was produced with the 9 kV high voltage and frequency of 11 kHz in all tests.
The Tektronix P6015A (wide bandwidth probe) was used for measuring the applied voltages. A digital phosphor oscilloscope (Tektronix DPO7104, 1 GHz bandwidth) was used for recording and observing the voltage and current waveforms.
The Optical Emission Spectrometer (OES) ranging between 200 and 1000 nm with the compact CCD spectrometer (CCS200 with the FWHM spectral accuracy of < 2 nm) was used for measuring the device spectrum. The spectra were subtracted from the dark baseline.
Animal samples
The ten weighted 25–30 g, 6-week old male Balb/c mice prepared from the Laboratory Animal Sciences Center of Baqiyatallah University, Iran, were used for in vivo blood coagulation tests. Another 25 mice with the same features were used for wound healing experiments. All the samples were kept under laboratory conditions, room temperature, atmospheric pressure and relative humidity of 18 ± 10%, 12 h night and 12 h lighting. They had free access to standard laboratory food and water. The experimental conditions were in compliance with the requirements of the Ethics Committee of the animal sciences center of Baqiyatallah University in Iran.
Treatment procedure
The animal tests were done in two phases: (1) the in vivo blood coagulation time investigation with non-thermal atmospheric pressure helium plasma jet, and (2) the determination of proper treatment time for skin wound healing. The ten mice were anesthetized by intraperitoneal injection of Ketamine/Xylazine, for blood coagulation tests. The mice were autopsied. Two cuts on the liver of each mouse were induced with the blazer as shown in Fig. 2. One of the cuts was allowed to coagulate naturally, another one was treated with helium plasma jet until it coagulated completely. The distance from the plasma device was 2 cm. The flow rate of helium gas was about 0.5 slm.
For wound healing tests, the 25 mice were assigned into 5 groups (10, 20, 30, 40, and 50 s treatment time group) with 5 animals in each group. The wounds, in all groups, were treated by helium plasma jet. The mice were anesthetized by intraperitoneal injection of Ketamine/Xylazine (100 mg/kg and 10 mg/kg body weight, respectively). Using a dermatology puncher, two circular 6 mm diameter wounds were inflicted on the left and right sides of the dorsal flank of each mouse. The left wound of each mouse was selected as wound that would be treated by non-thermal atmospheric pressure plasma and the right one as control wound that allowed to heal naturally (Fig. 3a). As the infected wounds are often thought to be stuck in the inflammation phase of the healing process, and they contain bacteria whose interaction with the surrounding tissue determines the healing rate of the wound [15], and this phase lasts 4–6 days [27], the wounds were treated for 5 consecutive days after wounding.
The mice were placed on the grounded plate. A typical mouse treated with helium plasma jet is shown in Fig. 3b.
Wound monitoring
The each wound area (control and treated wounds) was monitored at indicated days (1st, 3rd, 5th, 8th, and 10th days) by taking photographs of each wound in the direction perpendicular to the wound. The wound area were measured with the ImageJ code [28]. All of the wound measurements were done with one observer to reduce the uncertainty of measurements. The wound area reduction ratio values were obtained for each test group in respect to the wound area at first day for both control and treated wounds.
The wound area reduction ratio is defined as:
In which, (WR)j is the wound area reduction rate at day j, (WA)j is the wound area rate at day j and (WA)1 is the wound area at first day.
The percentages of wound area reductions against the control wound reductions for different days and for five groups have been obtained from the formula given in Eq. (2).
In which, R is the percentage of wound area reduction, (WR)j is the treated wound area reduction at day j, and (CWR)j is the control wound area reduction at day j.
Statistical analysis
The comparison between the time of blood coagulation treated by helium plasma jet and the naturally blood coagulation time was done with the one-way analysis of variance (ANOVA) using a least significance difference (LSD) test. Also, with in group comparisons of the wounds treated by helium plasma jet treated against the control wounds and the comparisons among 5 groups at each indicated day were done using ANOVA with LSD test. Data were reported as mean ± standard error. Also, p < 0.05 was considered statistically significant. All data were analysed used IBM SPSS statistics [29].
Histological analysis
On 10th day, 5 mice from each group (10, 20, 30, 40, and 50 s groups) were selected for histological analysis. Tissue samples were extracted from slaughtered specimens in the form of slices normal to each incision trajectory, both from the treated and control sectors. The slices were fixed in 10% buffered formalin for 24 h and then processed by histological techniques. The 5 µm thickness was sectioned and stained by hematoxilin-eosin. The samples were analysed with an optical microscope and with the magnification of ×40.
Results
Electric parameters of plasma jet
Figure 4 shows typical current and voltage waveforms of the He plasma jet.
The averaged instantaneous power resulting from multiplying voltage and current is 11.2 W.
Plasma OES spectroscopy result
Figure 5 shows the helium plasma jet emission spectrum in the wavelength range of 200–1000 nm. The reactive oxygen species (ROS) and reactive nitrogen species (RNS) are recognized in these spectra. OH and O which could play important role in the biomedical application such as blood coagulation and wound healing are observed in this spectrum. The process is carried out in the ambient air, so, the bands of the N2 (in the bands of 313–316 nm, 334–338 nm, 355–359 nm, 372–376 nm, 377–381 nm, 393–395 nm, 396–400 mm, 403–406 mm, 433–443 nm, 465–472 nm) are observed in this spectrum. OH is observed in the band of 306–310 nm, O at 377 nm, and N2+ at 427 nm. Also, the He peaks are observed in the spectrum as mentioned. The ROS and the RNS in non-thermal atmospheric pressure plasma have the ability to inactivate bacteria and improve oxygenation level in tissue [30]. OH radicals may activate redox reactions in tissue to influence the course of wound closure [18]. The existence of radicals in the plasma can cause repairing cell proliferation.
In vivo blood coagulation
Figure 6 shows a mouse liver treated by helium plasma jet. As shown in Fig. 6, the blood of the treated cut was coagulated completely and no blood oozed from the cut after the treatment. The wound remained wet. Meanwhile, the control cut continued to ooze until 10 min after cutting.
Figure 7 shows the 10 mice averaged in vivo coagulation time for both cuts (control and treated cuts). As shown, the averaged coagulation time for both control and treated cuts are 10 min and 8.6 s, respectively. The difference between two times is considerably significant (p < 0.001).
Skin morphology and wound monitoring results
Figure 8 shows the photographs of control and treated wound samples at indicated days (1st, 3rd, 5th, 8th, and 10th days) for 5 treatment time groups (10, 20, 30, 40, and 50 s).
As seen in Fig. 8, the scab started to form on the treated wounds at day 3 for all treatment time groups while the control ones were still inflamed. It can be seen that all the treated and the control wounds at the 1st day show the presence of a thin blood clot layer covered with exudation points. The wound healed from its borders towards the central zone at 5th day for treated wounds. At day 5, the scab on the treated wounds had started to fall off for 30 s, 40 s and 50 s groups. These process was seen at day 8 for 10 s and 20 s groups. The treated wounds had almost closed at day 8 for 30, 40, and 50 s groups, while, for 10 and 20 s groups, it happened at 10th day leaving only a narrow visible line. The scab has been seen on the control wounds by 10th day for all treatment time groups. There was not seen any side effects on the treated wounds and wound surroundings.
Figure 9 shows the comparison between wound area reduction ratios in respect to the mentioned days (3rd, 5th, 8th, and 10th days) for different treatment time groups (10, 20, 30, 40 and 50 s). Also. The comparison between the control wound area reduction ratios and treated wound area reduction ratios has been reported.
As seen in Fig. 9, the treated wound area reduction ratios are more than the control ones for all treatment time groups and for each monitoring day. For 10 and 20 s treatment time groups, it is not seen significant differences between different days (p > 0.05). For 30, 40, and 50 s treatment time groups the treated wound area reductions ratios are more than the control ones in all days. For 30 s group, the differences between control and treated wound area reductions are more significant than the other groups.
Figure 10a, b, c show the comparison between the wound area reduction rations between each pair of groups in 3rd, 5th, and 8th days, respectively.
As shown, at 3rd, 5th, and 8th days, the differences between groups 10 and 30 s, 10 and 40 s, 10 and 50 s, 20 and 30 s, 20 and 40 s, and 20 and 50 s are statistically significance (p < 0.05). The differences between among the other groups in days 3rd, 5th, and 8th and between all groups in 10th day are not significant (p > 0.05). It is concluded from these results that the 30, 40, and 50 s treatment times show the significant differences on wound area reduction ratio values. Given that, it is desirable to impose lower dose to the living tissue, among three treatment time groups (30, 40, and 50 s), 30 s is chosen as proper treatment time.
Figure 11 shows the comparison between the treated wound area reduction rations in respect to the observation days for each group.
As it is observed, in all groups the differences between the wound area reduction ratios in day 3 and day 5 aren’t important. The differences between the day 3 and day 8 are important for all groups, but these differences are more important for 30 s, 40 s and 50 s groups (p < 0.001). The differences between day 3 and day 10, day 3 and day 12, day 5 and day 8, day 5 and day 12 are important for all groups. The differences between day 8 and day 10, day 8 and day 12 at 10 s and 20 s groups are important. The differences among other days are not significant in all groups (p > 0.05). So, it is concluded that the wounds treated by plasma jet show more fast healing at first days than the last days. For 10 s and 20 s groups, the differences among first days, for example day 3 and day 5, day 5 and day 8 are less than the differences among the last day 8 and day 10, day 8 and day 12. These show that the healing of the wounds at first days is less than that of the last days. For 30 s, 40 s and 50 s groups, the differences between first days like day 3 and day 8, and day 5 and day 8 are more important than the differences between the last days like day 8 and 10, and day 8 and 12. These show that the healing process in first days is faster than that of for last days. It concluded that the treatment for 30 s, 40 s and 50 s by non-thermal plasma shows faster healing than that of for 10 s and 20 s groups.
Table 1 shows the percentages of wound area reduction ratios against the control ones for different days and for 5 groups.
As seen in Table 1, the percentages of wound area reduction ratios for 30 s treatment time is 78% at 3rd day. At 5th day and 8th day, these values are 77% and 63%, respectively. For 40 s group, these values are 77%, 70% and 58% at 3rd, 5th and 8th days, respectively. These values for 50 s group are 76%, 74% and 64%, respectively. The inflammatory phase which is the body’s natural response to injury lasts 4–6 days [30] from the injury event. The probability of bacterial contamination exists in these days, so, the treatment in these days is very important. As concluded from Table 1, the 30, 40, and 50 s treatment time groups have the most effects on the wound healing at first days.
Histology assessment
Figure 12 shows the histological microscopy images of tissue samples for the untreated control wounds and the wounds treated with different treatment times (10, 20, 30, 40, and 50 s) by non-thermal plasma at 10th day.
Histological microscopy images of the wound tissue samples: a control wound (×40 magnification), b wound treated for 10 s (×40 magnification), c wound treated for 20 s (×40 magnification), d wound treated for 30 s (×40 magnification), e wound treated for 40 s (×40 magnification), and f wound treated for 50 s treatment time (×40 magnification)
As seen in Fig. 12a, for untreated control wound, the epidermis made bridge at cutting edge by new layer of epithelial cells, but not well-organized and the subepithelial layer filled by a thin layer of connective tissue and hat epidermal cell regeneration was not complete in the control wound tissue. For wound treated 10 s by helium plasma jet, the same effect as control wound was observed, but the epidermis layer was more thickened. For wound treated 20 s by helium plasma jet, the wound edges were bridged by a new layer of epithelial cell but it was very thin and not well-organized. For 30 s and 40 s treated wounds, the regeneration of epidermis as well as the inflammatory phase was finished and collagen deposition was observed. The epidermis layers for both groups were thicker than others. The regeneration of epidermis was finished. For 50 s group, the epidermis layer was develop but not well-organized and dermis has not developed, yet. These results indicated that the wounds treated with 30 s and 40 s for 5 consecutive days by Helium plasma jet were healed faster and better than the other groups.
It is concluded from morphological and histological analysis (Figs. 8, 10; Table 1) that the 30 and 40 s groups are appropriate than the other ones. Nevertheless, among these groups, 40 s group impose more plasma dose to living tissue than the 30 s treatment time group. As a lower treatment time is useful to reduce patient discomfort, the 30 s have been chosen as the proper treatment time.
Discussions
Blood coagulation is, in general, a complex process that involves platelets, various coagulation proteins, and ions. Some simplified models of the coagulation cascade indicate the role of calcium ions. In fact, the most reactions in the coagulation cascade depend on calcium ion concentration. In vivo, platelet activation usually initiates the coagulation cascade leading to platelet aggregation and then conversion of fibrinogen into cross-linked fibrin occurs, binding platelets to form a clot. Non-thermal plasma may coagulate blood through multiple pathways including platelet activation, activation of intermediate protein factors and increasing concentration of ionic species [14, 31, 32]. In this research, the in vivo blood coagulation of the liver cut of the mice was investigated by atmospheric pressure plasma treatment. As shown in Fig. 6, the blood of the treated cut was coagulated completely and no blood oozed from the cut after the treatment. It was obtained that the averaged coagulation time for both control and treated cuts are 10 min and 8.6 s, respectively.The skin is the largest organ of the body. It has three main layers, the epidermis, the dermis and the subcutaneous layer. The healing of skin wounds progresses through sequential and overlap** phases of homeostasis, inflammation, proliferation, and maturation. Each phase of healing is directed by the complex coordination and interaction of several cell types contained within the wound, including inflammatory cells such as neutrophils, macrophages, lymphocytes, and platelets. Native skin cells such as fibroblasts, keratinocytes, and vascular endothelial cells are also intricately involved in these processes. The inflammatory phase of wound repair is characterized by formation of a fibrin clot, activation of platelets with subsequent release of platelet-derived factors, and an influx of macrophages followed closely by lymphocytes. The activation of circulating and resident cell populations results in the release of soluble mediators such as tumor necrosis factor-α (TNF-α), interleukins, nitric oxide (NO), and growth factors. In addition, epithelial closure (epithelialization) and wound contraction begin in this early stage. Epithelialization is critical for wound closure and is dependent on both proliferation and migration. The granulation tissue formation or proliferative phase involves nearly every cell type in the skin. Fibroblasts from the surrounding dermis migrate into the injured site and are responsible for contraction, proliferation. The remodelling or maturation phase involves continued proliferation of fibroblasts and deposition of newly synthesized matrix (termed fibroplasia), completed neovascularization, and formation of mature scar. As reported, the bacterial infection of wound may happen in the inflammatory phase and it may prevent the wound healing process from entering the proliferative phase and can even cause damage to the extracellular matrix. The ROS and RNS produced by atmospheric pressure cold plasma can inactivate bacteria and improve the blood oxygenation level in tissue. As shown in Fig. 5, the ROS and RNS were observed in the optical emission spectrum, in this research. For example, the OH radical that may activate redox reactions in tissue to influence the course of wound closure was seen in the spectrum. The existence of the other radicals in the plasma can cause repairing cell proliferation. As shown in Figs. 8, 9, and 10, the treatment of the wound by the non-thermal atmospheric pressure plasma for 5 consecutive days, can accelerate the regeneration and maturation of epidermis and cause the treated wounds to heal faster than untreated control wounds in all groups. It is concluded from Fig. 11 that the plasma affected on the wound healing and accelerates wound healing process at first days in all groups. As shown in Fig. 12, for 30 s and 40 s treated wounds, the regeneration of epidermis as well as the inflammatory phase was finished and collagen deposition was observed. The epidermis layers for both groups were thicker than others. The regeneration of epidermis was finished. For 50 s group, the epidermis layer was develo** but not well-organized and dermis has not developed, yet. These results indicated that the wounds treated with 30 s and 40 s for 5 consecutive days by Helium plasma jet were healed faster and better than the other groups. Among these groups, 40 s group impose more plasma dose to living tissue than the 30 s treatment time group. As a lower treatment time is useful to reduce patient discomfort, the 30 s have been chosen as the proper treatment time.
Conclusions
The focus of this article was on determining the proper treatment time for blood coagulation and wound healing of the Balb/c mice. For this reason, a plasma jet device working in helium gas was used. In these animal tests, the in vivo cuts on the 10 mice livers showed the averaged coagulation time of 8.6 s. The cut bleeding stops after 10 min, naturally. The investigation on the effect of different treatment times on wound healing showed that the 30 s and 40 s exposures in 5 consecutive days using non-thermal atmospheric pressure helium plasma jet resulted in the fastest and best treatment procedure according to the morphological and histological analysis. As the inflammatory phase of wound healing that the bacterial infection of wound may happen in is 4–5 days, the 5 days of treatment was chosen. It is observed that the 30, 40, and 50 s treatment times for 5 consecutive days show the significant differences on wound area reduction ratio values. The percentages of wound area reduction ratio values against the control ones for 30 s treatment time is 78% at 3rd day, 77% at 5th day and 63% at 8th day, respectively. For 40 s and 50 s groups, these values are 77%, 70%, 58%, and 76%, 74%, 64%, respectively. The averaged values of wound area reduction ratio in 30 s treatment time group are more than the others. Among the three groups (30 s, 40 s and 50 s), for 50 s group the epidermis layer was develo** but not well-organized and dermis has not developed but for 30 s and 40 s groups the regeneration of epidermis was finished and collagen deposition was observed. Among two groups (30 s and 40 s), 40 s treatment time impose more plasma dose to living tissue than the 30 s treatment time group, so, the 30 s have been chosen as the proper treatment time for wound healing.
References
Howatson AM (1976) An introduction to gas discharge, 2nd edn. Academic press, Cambridge
Fridman A, Chirokov A, Gutsol A (2005) Non-thermal atmospheric pressure discharges. J Phys D 38:R1–R24
Laroussi M et al (2000) Biological decontamination by nonthermal plasmas. IEEE Trans Plasma Sci 28:184–188
Fridman G, Peddinghaus M, Balasubramanian M, Ayan H, Fridman A, Gutsol A, Brooks A (2006) Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem Plasma Process 26:425
Stoffels E (2000) Biomedical applications of electric gas discharge. High Temp Mater Process (New York) 5(2):191–202
Graves DB (2014) Low temperature plasma biomedicine: a tutorial review. Phys Plasmas 21:080901
Benstaali B et al (2002) Density and rotational temperature measurements of the OH and NO radicals produced by a gliding arc in humid air. Plasma Chem Plasma Proc 22:553–571
Benstaali B et al (1998) Plasma treatment of aqueous solutes: some chemical properties of a gliding arc in humid air. Eur Phys J Appl 4:171–179
Laroussi M, Leipold F (2004) Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom 233:81–86
Heinlin J, Morfill G, Landthaler M et al (2010) Plasma medicine: possible applications in dermatology. J Dtsch Dermatol Ges 8(12):968–976
Kolb JF, Mohamed AAH, Price RO, Swanson RJ, Bowman A, Chiavarini RL, Stacey M, Schoenbach KH (2008) Cold atmospheric pressure air plasma jet for medical applications. Appl Phys Lett 92:241501
Uhm HS, Lim JP, Li SZ (2007) Sterilization of bacterial endospores by an atmospheric-pressure argon plasma jet. Appl Phys Lett 90:26150
Weidinger A, Kozlov AV (2015) Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules 5(2):472–484
Silverthorn DU (2006) Human physiology, 4th edn. Pearsons Education, Inc., London, p 552
Agyingi E, Maggelakis S, Ross D (2010) The effect of bacteria on epidermal wound healing. Math Model Nat Phenom 5(3):28–39
Janani E, Ale-Ebrahim M, Mortazav P (2013) In vitro and in vivo studies of the effects of cold argon plasma on decreasing the coagulation time. Iran J Med Phys 10:31–36
Miyamotol K, Ikehara S, Takei H, Akimoto Y et al (2016) Red blood cell coagulation induced by low-temperature plasma treatment. Arch Biochem Biophys 605:95–101
Xu G-M, Shi X-M, Cai J-F, Chen S-L, Yao C-W, Chang Z-S, Zhang G-J, Li P (2015) Dual effects of atmospheric pressure plasma jet on skin wound healing of mice. Wound Repair Regen 23(6):878–884
Dowling DP, McDonnell KA, Chebbi A, Breathnach R (2015) Use of a rat model to demonstrate an enhanced rate of wound healing obtained using atmospheric plasma treatments. In: 22nd International Symposium on Plasma Chemistry, Antwerp, Belgium, July 5–10, 2015
García-Alcantara E, López-Callejas R, Morales-Ramírez PR, Peña-Eguiluz R, Fajardo-Muñoz R, Mercado-Cabrera A, Barocio SR, Valencia-Alvarado R, Rodríguez-Méndez BG, Muñoz-Castro AE, de la Piedad-Beneitez A, Rojas-Olmedo IA (2013) Accelerated mice skin acute wound healing in vivo by combined treatment of argon and helium plasma needle. Arch Med Res 44(3):169–177
Salehi S, Shokri A, Khani MR, Bigdeli M, Shokri B (2015) Investigating effects of atmospheric-pressure plasma on the process of wound healing. Biointerphases 10(2):029504
Fathollah S, Mirpour S, Mansouri P, Dehpour AR, Ghoranneviss M, Rahimi N, Naraghi ZS, Chalangari R, K.M. Chalangari (2016) Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rat. Sci Rep 6:19144
Shahbazi Rad Z, Abbasi Davani F (2016) Non-thermal atmospheric pressure dielectric barrier discharge plasma source construction and investigation on the effect of grid on wound healing application. Clin Plasma Med 4:56–64
Shahbazi Rad Z, Abbasi Davani F (2017) Experimental investigation on electrical characteristics and dose measurement of dielectric barrier discharge plasma device used for therapeutic application. Rev Sci Instrum 88(4):043504
Nakajima Y, Mukai K et al (2014) Cold plasma on full thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin Plasma Med 2:28–35
Nastuta AV, Topala I, Grigoras C et al (2011) Stimulation of wound healing by helium atmospheric pressure plasma treatment. J Phys D 44:105204
http://www.Pilonidal.org/surgery-aftercare/wound-healing-in-depth/
Qiu H, ** GQ, ** RF, Zhao WK (2007) Analysis of variance of repeated data measured by water maze with SPSS. J Chin Integr Med 5:101–105
http://www.clinimed.co.uk/Wound-Care/Education/Wound-Essentials/Phases-of-Wound-Healing.aspx
Ataullakhanov FI, Pohilko AV, Sinauridze EL, Volkova R (1994) Calcium threshold in human plasma clotting kinetics. Thromb Res 75(4):383–394
Butenas S, Veer C, Mann K (1999) Normal thrombin generation. Blood 94(7):2169–2178
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The research hasn't any conflict of intensest financially or non-financially. The experimental tests on animals were in compliance with the requirements of the Ethics Committee of the animal sciences center of Baqiyatallah University.
Rights and permissions
About this article
Cite this article
Shahbazi Rad, Z., Abbasi Davani, F. & Etaati, G. Determination of proper treatment time for in vivo blood coagulation and wound healing application by non-thermal helium plasma jet. Australas Phys Eng Sci Med 41, 905–917 (2018). https://doi.org/10.1007/s13246-018-0686-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-018-0686-z