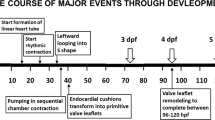
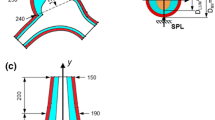
Abstract
Several studies have linked abnormal blood flow dynamics to the formation of congenital heart defects during the early stages of development. The objective of this study is to document the transition of pum** mechanics from the early tube stage to the late loo** stage of the embryonic heart. The optically transparent zebrafish embryonic heart was utilized as the in vivo model and was studied using standard bright field microscopy at three relevant stages within the transitional period: (1) tube stage at 30 hours post-fertilization (hpf); (2) early cardiac loo** stage at 36 hpf; and (3) late cardiac loo** stage at 48 hpf. High-speed videos were collected at 1000 fps at a spatial resolution of 1.1 μm/pixel at each of these stages and were post-processed to yield blood velocity patterns as well as wall kinematics. Results show that several relevant trends exist. Morphological trends from tube through late loo** include: (a) ballooning of the chambers, (b) increasing constriction at the atrioventricular junction (AVJ), and (c) repositioning of the ventricle toward the side of the atrium. Blood flow trends include: (a) higher blood velocities, (b) increased AVJ regurgitation, and (c) larger percentages of blood from the upper atrium expelled backward toward the atrial inlet. Pum** mechanics trends include: (a) increasing contraction wave delay at the AVJ, (b) the AVJ begins acting as a rudimentary valve, (c) decreasing chamber constriction during maximum contraction, and (d) a transition in ventricular kinematics from a pronounced propagating wave to an independent, full-chamber contraction. The above results provide new insight into the transitional pum** mechanics from peristalsis-like pum** to a displacement pum** mechanism.







Similar content being viewed by others
References
Auman, H. J., et al. Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol. 5(3):604–615, 2007.
Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 91(2):279–288, 2011.
Bartman, T., et al. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2(5):673–681, 2004.
Beis, D., and D. Y. R. Stainier. In vivo cell biology: following the zebrafish trend. Trends Cell Biol. 16(2):105–112, 2006.
Beis, D., et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132(18):4193–4204, 2005.
Bhat, S., J. Ohn, and M. Liebling. Motion-based structure separation for label-free high-speed 3-D cardiac microscopy. IEEE Trans. Image Process. 21(8):3638–3647, 2012.
Broekhuizen, M. L. A., et al. Altered hemodynamics in chick embryos after extraembryonic venous obstruction. Ultrasound Obstet. Gynecol. 13(6):437–445, 1999.
Butcher, J. T., et al. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ. Res. 100(10):1503–1511, 2007.
Chico, T. J. A., P. W. Ingham, and D. C. Crossman. Modeling cardiovascular disease in the zebrafish. Trends Cardiovasc. Med. 18(4):150–155, 2008.
Dahme, T., H. A. Katus, and W. Rottbauer. Fishing for the genetic basis of cardiovascular disease. Dis. Models Mech. 2(1–2):18–22, 2009.
Filas, B. A., I. R. Efimov, and L. A. Taber. Optical coherence tomography as a tool for measuring morphogenetic deformation of the loo** heart. Anat Rec. Adv. Integr. Anat. Evol. Biol. 290(9):1057–1068, 2007.
Forouhar, A. S., et al. The embryonic vertebrate heart tube is a dynamic suction pump. Science 312(5774):751–753, 2006.
Harvey, R. P. Patterning the vertebrate heart. Nat. Rev. Genet. 3(7):544–556, 2002.
Hogers, B., et al. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ. Res. 80(4):473–481, 1997.
Hogers, B., et al. Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc. Res. 41(1):87–99, 1999.
Hove, J. R., et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421(6919):172–177, 2003.
Jamison, R. A., A. Fouras, and R. J. Bryson-Richardson. Cardiac-phase filtering in intracardiac particle image velocimetry. J. Biomed. Opt. 17(3):7, 2012.
Johnson, B., D. Garrity, and L. P. Dasi. Quantifying cardiac function in the embryonic heart. J. Biomech. Eng. Trans. ASME, 2013, accepted for publication.
Kimmel, C. B., et al. Stages of embryonic-development of the zebrafish. Dev. Dyn. 203(3):253–310, 1995.
Kopp, R., B. Pelster, and T. Schwerte. How does blood cell concentration modulate cardiovascular parameters in develo** zebrafish (Danio rerio)? Comp. Biochem. Physiol. Mol. Integr. Physiol. 146(3):400–407, 2007.
Liebling, M., et al. Rapid three-dimensional imaging and analysis of the beating embryonic heart reveals functional changes during development. Dev. Dyn. 235(11):2940–2948, 2006.
Lieschke, G. J., and P. D. Currie. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 8(5):353–367, 2007.
Liu, A.P., et al. Biomechanics of the chick embryonic heart outflow tract at HH18 using 4D optical coherence tomography imaging and computational modeling. PLoS One 7(7), 2012.
Loumes, L., I. Avrahami, and M. Gharib. Resonant pum** in a multilayer impedance pump. Phys. Fluids 20(2):11, 2008.
Manner, J., A. Wessel, and T. M. Yelbuz. How does the tubular embryonic heart work? Looking for the physical mechanism generating unidirectional blood flow in the valveless embryonic heart tube. Dev. Dyn. 239(4):1035–1046, 2010.
Roman, B. L., et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 129(12):3009–3019, 2002.
Scherz, P. J., et al. High-speed imaging of develo** heart valves reveals interplay of morphogenesis and function. Development 135(6):1179–1187, 2008.
Stainier, D. Y. R., and M. C. Fishman. The zebrafish as a model system to study cardiovascular development. Trends Cardiovasc. Med. 4(5):207–212, 1994.
Stainier, D. Y. R., R. K. Lee, and M. C. Fishman. Cardiovascular development in the zebrafish. 1. Myocardial fate map and heart tube formation. Development 119(1):31–40, 1993.
Thisse, C., and L. I. Zon. Development—organogenesis—heart and wood formation from the zebrafish point of view. Science 295(5554):457–462, 2002.
Westerfield, M. The Zebrafish Book. Eugene, OR: University of Oregon Press, 1995.
Acknowledgments
We acknowledge funding from the National Science Foundation (Award# 1235305), program director Dr. Dennis Carter. We also thank Dr. Lindsay Parrie and Dr. Yelena Chernyavskaya for sharing their expertise regarding zebrafish preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Kerem Pekkan and Bradley B. Keller oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Johnson, B.M., Garrity, D.M. & Dasi, L.P. The Transitional Cardiac Pum** Mechanics in the Embryonic Heart. Cardiovasc Eng Tech 4, 246–255 (2013). https://doi.org/10.1007/s13239-013-0120-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-013-0120-3