Abstract
The aim of this study is to convert polypropylene waste into usable liquid fuel via pyrolysis technique using kaolin as a low-cost catalyst. Waste polypropylene was thermally and catalytically degraded in a chemical vapour deposition (CVD) horizontal glass reactor at a temperature of 450 °C, residence time of 30 min, and heating rate of 30 °C/min. The kaolin clay was characterized by XRF analysis while the ultimate and proximate analysis of the polypropylene feed carried out gave combustible materials content of 93.77 wt%, fixed carbon of 1.62 wt%, calorific value of 45.20 MJ/kg and elemental composition with carbon (83.65%), hydrogen (14.27%), oxygen (0.15%), sulphur (0.1%), chlorine (1.16%), and nitrogen (0.67%). Thermal cracking was carried out in the absence of catalyst and the process gave a yield of liquid, gaseous, and solid products of 67.48, 8.85, and 23.67 wt%, respectively. Furthermore, kaolin clay was employed as a catalyst in catalytic pyrolysis of the same feedstock for catalyst-to-plastic ratio of 1:1, 1:2, 1:3, and 1:4 at the same operating parameters as in thermal cracking. Optimum yield was obtained at a catalyst-to-plastic ratio of 1:3 with a yield of 79.85, 1.48, and 18.67 wt% for liquid, gaseous, and solid products, respectively. The liquid products obtained for both thermal and catalytic cracking at optimum conditions were characterized for their suitability as fuel. The properties determined were density, viscosity, flash point, fire point, pour point, and calorific value. The results suggest that catalytic pyrolysis produced liquid products, whose properties are comparable to conventional fuels (gasoline and diesel oil) than that produced through thermal pyrolysis. FTIR analysis of the liquid product from catalytic pyrolysis also shows that it contains hydrocarbons with different functional groups such as aromatics, olefins, carbonyl, amines, sulphides, and hydroxyl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a growing interest in producing fuel which can serve as an alternative to fossil fuel as a result of concerns arising from green house gas emissions and consequent threat to human life [1]. Generating fuel from other sources aside fossil resources can complement the nonrenewable fossil fuel reserves [2]. Latest research focus is now on production of fuel from biomass. Presently, biofuels are produced from bio-matter, mostly edible plant matter which serves as food for man using what was termed ‘first generation’ technologies; the use of which reduces man’s food reserve [3]. Second generation technologies for the production of biofuels allow the use of nonedible cellulosic matter which includes: agricultural residues (straw, grain husks, plant matter, etc.), domestic and industrial wastes (paper, glass, plastics, metal scraps, gabbage, etc.), and some specially grown energy crops such as Neem, Jathropha, and Moringa [2, 4].
Plastic materials have presently found wide applications and acceptance in the production of domestic household utensils, in automobile parts industry and several other manufacturing processes [5]. The huge quantities of these plastic products will definitely end up in waste dump sites due to their non biodegradable nature, thus increasing the quantity of solid waste generated globally [6]. Waste management and utilisation has been a global concern and as such with the current global focus on conversion of waste to useful energy resources (wtE), there is a concerted call to look for efficient and effective ways of converting these wastes into useful energy resources [7].
Pyrolysis has been identified as an efficient means of producing liquid fuel from municipal waste [7,8,9]. It is a thermochemical conversion process that requires the application of heat in the temperature range of 250–600 °C [10]. According to Bulushev and Ross [2], the process is usually carried out under inert atmosphere, resulting into the production of liquid, gaseous, and solid fuels. The proportion of the products formed are dependent on many factors such as nature of catalyst used, temperature of pyrolysis, starting raw material, mode, and type of pyrolysis system employed. These products can be used mainly as blending components for fuels or as additives for the production of valuable chemicals if it cannot be used as a perfect replacement for products of fossil fuels [3, 7, 11].
The use of catalysts in thermal degradation (pyrolysis) of plastics has been known to result in quantitative and qualitative improvement in the liquid fuel yield [6, 12]. In recent years, synthetic catalysts such as zeolites, silica alumina, zinc oxides, calcined dolomite, and synthesized fly ash have been in use for catalytic pyrolysis. However, these synthetic catalysts are not readily available and it comes with high cost of production, thus making catalytic cracking relatively expensive [13].
A number of studies have been documented on the catalytic pyrolysis of different plastic materials and other biomass for energy generation. Sonawane et al. [8] carried out pyrolysis of polypropylene in the presence of 10% NZ catalyst; Bulushev and Ross [2] cracked HDPE over silica alumina catalyst; Khaing and Chaw [4] catalytically degraded mixed plastic waste (PP, HDPE, LDPE, PS, and PET) using zinc oxide catalyst; Dimitris and Achilias [12] used Ni/HSiAl catalyst for the pyrolysis of mixed plastic wastes; Tursunov [1] compared the catalytic efficiency of zeolite and calcined dolomite for the pyrolysis of MSW; Ates et al. [3] used Y-zeolite, β-zeolite, FCC, MoO3, Ni–Mo, HZSM-5, and Al(OH)3 as catalysts for thermal degradation of MPW; Satyendra et al. [7] used coal fly ash as a catalyst in cracking HDPE; and Panda and Singh [14] used a commercial grade kaolin for the pyrolysis of PP. Nearly, in all these studies, synthetic and analytical grade catalysts were used for the production of fuels. These catalysts are relatively expensive. The need for a cheaper local alternative has become imperative.
No previous attempt has been made in exploring the potential of Ahoko clay in Kogi state of Nigeria as a catalyst for the pyrolysis of plastic waste; thus, this study is aimed at verifying its potential as a catalyst for the pyrolysis of waste polypropylene into usable fuel. The use of kaolin from Ahoko clay will reduce significantly the cost associated with catalytic pyrolysis from the use of synthetic and analytical grade catalysts. Ahoko clay is naturally abundant and has been reported by Kovo [15] to contain Si/Al in a ratio which is very close to that of zeolites and has high mesoporous surface area, excellent acidic properties and large pore volume. These properties make it an excellent material for catalytic pyrolysis.
Methodology
Materials
Polypropylene plastic wastes of drinking straws, disposable cups and spoons, margarine containers, custard containers, and yoghurt containers were employed as raw materials for the pyrolysis process together with Ahoko kaolin clay as a catalyst.
Methods
The plastic wastes were sorted, washed, dried, and shredded into small sizes. Ultimate and proximate analysis of a sample of the prepared feedstock was undertaken. Ahoko kaolin was characterized by X-ray fluorescence (XRF).
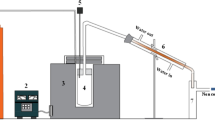
The pyrolysis equipment comprised of a horizontal chemical vapour deposition (CVD) reactor with a glass tube and a condenser fabricated with borosilicate glass which was fitted to the reactor at one end and constant supply of nitrogen as inert gas was passed through the other end. The whole equipment was fitted to a furnace with a maximum heating temperature of 1200 °C. The complete equipment setup is shown in Fig. 1.
Thermal and catalytic cracking
Waste polypropylene was thermally degraded in a CVD reactor by heating a given amount of the prepared feedstock in the absence of catalyst. Pyrolytic condition was established by purging nitrogen gas from a gas cylinder into the reactor to drive away any air or oxidative matter present in the system. The pyrolysis was undertaken at a preset temperature of 450 °C and residence time of 30 min which were the averages of the reported temperature and time ranges for pyrolysis by [8, 16] who both reported 350–550 °C and 15–45 min as pyrolysis temperature and residence time ranges, respectively. Most of the condensable vapour condensed through the condenser into liquid fuel which was collected into a beaker at the other end. The liquid fuel collected in the beaker was weighed, solid residue scrapped from the reactor and weighed, and then, the gaseous product was obtained from material balance. The whole process was repeated but now in the presence of Ahoko kaolin as a catalyst for different catalyst-to-plastic ratios of 1:1, 1:2, 1:3, and 1:4. In each case, the liquid, gaseous, and solid yields were determined. The yields of the fractional products were calculated separately. The mass balance yields were calculated as the ratio of the corresponding measurable product phase (liquid and solid products) to the initial feedstock mass. The gas yield was equally calculated based on the resulting mass difference according to Gao [17], Sharma et al. [18].
Characterization of the liquid fuel
The liquid product obtained from both the thermal and catalytic pyrolysis at optimum condition (catalyst-to-plastic ratio 1:3) was characterized for some of its physical properties, namely, density, viscosity, flash point, fire point, calorific value, and pour point according to American Standard for Testing and Materials (ASTM). Fourier Transformed Infrared Spectrometry (FTIR-S) characterization of the oil was also carried out to determine the functional groups present in the oil using SHIMADZU FTIR-8400S machine.
Results and discussion
Proximate and ultimate analysis of waste polypropylene feed
The result of the proximate and ultimate analysis of the waste PP is presented in Table 1.
The combustible content is the quantity of matter that can be thermally degraded in a given substance. This is a measure of the amount of matter that can be recovered upon cracking [18]. High combustible content indicates high energy and matter recovery from a material. The combustible content of the polypropylene feedstock is high enough (93.77%). This result indicates that high amount of products can be obtained upon thermal cracking. This value is in close range to most reported work; 89.65% by Panda [19], 91.48% by Khaing and Chaw [4], and 94.59% by Gao [17]; however, the value obtained was significantly different from the study of Sonawane et al. [8] with a reported value of 83.56%. This considerable variation can be attributed to differences in the polymeric material used. Other properties determined were in consonance to most documented work on the proximate analysis of plastic materials [20, 21]).
The ultimate analysis result, as presented in Table 1 (column 2), shows the suitability of polypropylene as feedstock for the pyrolysis process based on its high carbon value. The presence of oxygen, sulphur, chlorine, and nitrogen might be attributed to additives added during polymer processing. The presence of sulphur in particular can be traced back to natural gas which is the starting raw material for the polymerization of propylene to polypropylene. The ratio of carbon to hydrogen obtained in this study is as expected judging from the chemical composition of propylene; C3H6, the % weight of carbon is about 85.71 and that of hydrogen is around 14.29 which are very close to the obtained value of 83.65 and 14.27% for carbon and hydrogen, respectively. The little variation might be due to the presence of other elements as revealed by the ultimate analysis results.
X-ray fluorescence (XRF) analysis of kaolin catalyst
XRF analysis of the kaolin clay revealed the presence of SiO2 (72.26%), Al2O3 (18.96%), Fe2O3 (1.05%), MgO (0.13%), MnO (0.004%), K2O (0.431%), Na2O (0.021%), TiO2 (1.485%), and Loss on Ignition (LOI) at 1000 °C as 6.17%. The result indicated that the major compounds present in kaolin were alumina (Al2O3) and silica (SiO2), and this is expected, because the major constituent of sand for which clay is a type are silica and alumina. Variation in the ratio of silica to alumina is the basis for different types of clay [22]. The presence of other metal oxides is as a result of special properties of kaolin which make it gain wide acceptance as a catalyst. The silica alumina ratio (SiO2/Al2O3) is 3.8. Panda and Singh [14], characterized commercial kaolin obtained from Chemtex Corporation, Kolkata, India, and reported 43.12% SiO2, 46.07% Al2O3, nil Fe2O3, 0.027% MgO, 0.030% CaO, 0.0064% ZnO, 0.001% K2O, 0.74% TiO2, and 9.9% LOI at 1000 °C. Most of the values reported are in close range to the one obtained in this study. A significant difference of about 20% was observed for both the silica and alumina values. This considerable difference can be ascribed to the distinction in the grades of the kaolin characterized. Furthermore, Aroke and El-Nafty [23] characterized modified organo-kaolinite clay obtained from Alkaleri, Bauchi state, Nigeria and reported 36.490% SiO2, 56.290% Al2O3, 0.080% P2O5, 0.596% K2O, 0.141 CaO, 3.438% TiO2, 0.22% CuO, among others. The reported results are in close proximity to the results obtained in this work. Observable differences can be largely tied to the treatment and modification process of the kaolin used.
Thermal and catalytic pyrolysis of waste PP
The products’ yields for both the thermal and catalytic pyrolysis are presented in Tables 2 and 3, respectively.
For thermal cracking, the first drop of liquid from the condenser was observed at a temperature of 150 °C, and pure polypropylene melts at a temperature of 135 °C [16]. The 15 °C discrepancy might be due to the presence of additives added during polymer processing which might have increased the melting point of polypropylene. However, for catalytic pyrolysis, the first drop of liquid from the condenser was noticed at a temperature of 140 °C which is very close to the melting temperature of pure polypropylene (135 °C). This improvement results from the increased cracking rate of the plastic due to the presence of kaolin catalyst. The products’ yield for the thermal cracking, as shown in Table 2, was 67.48 wt% for liquid, 8.85 wt% for gas, and 23.67 wt% for solid products. For the catalytic cracking, the liquid yield increases with increase in catalyst-to-plastic ratio up to 1:3, the solid product decreases with increase in catalyst-to-plastic ratio, while the gaseous product also increases with increase in catalyst-to-plastic ratio. The highest product yield was obtained at a catalyst-to-plastic ratio of 1:3 with liquid yield of 79.85 wt%, gaseous yield of 18.67 wt%, and solid product yield of 1.48 wt%. This increase in liquid yield during catalytic cracking is a substantial improvement over thermal cracking due to the catalytic nature of kaolin which aided the cracking process. The behaviour in the cracking process in the presence of kaolin as a catalyst can be explained by its mesoporous surface area, acidity, and high Si/Al ratio which facilitated the cracking reaction.
The liquid products’ yield increased from 69.75 (wt%) to 79.85 (wt%) when catalyst-to-polypropylene ratio was increased from 1:1 to 1:3. Thus, an increment of about 10 (wt%) in oil yield was observed for an increment in catalyst-to-plastic ratio of 1:3. However, further increase in catalyst-to-plastic ratio to 1:4 led to a decrease in the oil yield to 76.49 (wt%), equivalent to about 3% decrease. This behaviour might be due to the high plastic-to-catalyst ratio as the catalyst effect is no longer significant for the process, thus decreasing the yield of liquid product and increasing the gas and char yields. The presence of catalyst in the ratio 1:3 must be significant for optimum product yield. Furthermore, from Table 3, it can be observed that the char yield showed an interesting pattern with slight decrease between ratio 1:1 and 1:2 (from 14.01 to 10.42 wt%); however, beyond this ratio up to ratio 1:3, abrupt decrease was observed (from 10.42 to 1.48 wt%). This behaviour can be attributed to the heavy cracking of the plastic by the catalyst which was observed at ratio 1:3 as confirmed by the highest liquid product yield at that ratio. Furthermore, beyond the optimum ratio, the char yield experienced a gradual increase (from 1.48 to 1.99 wt%) and a drop in the liquid product which is indicative of a reduction in catalyst activity. Gaseous products’ yields increased steadily throughout the process with about 1.5 wt% increment across each catalyst-to-plastic ratio. This implies that the catalyst has virtually no effect on the gaseous products.
Congruent results were obtained by Panda and Singh [6] when polypropylene was thermally decomposed in the presence of commercial grade kaolin at various catalyst-to-plastic ratios of 1:1, 1:2, 1:3, 1:4, 1:5, 1:6 up to 1:20. The effect of catalyst in the process was significant up to catalyst-to-plastic ratio of 1:5, but at a ratio of 1:10, the effect was almost insignificant on the products’ yield and at the extreme ratio of 1:20, the effect of catalyst was inconsequential on the products’ yield. Optimum product yield was obtained at a catalyst-to-plastic ratio of 1:3. This behaviour was attributed to imbalance in the catalyst-to-plastic ratio; thus, it was recommended that the amount of catalyst used in catalytic pyrolysis should be significant enough but not more than the feedstock to be cracked.
Determination of physio-chemical properties of the liquid fuel
The physio-chemical properties of the liquid products obtained from thermal and catalytic pyrolysis at optimum conditions are determined and are shown in Table 4.
The liquid fuels obtained from both thermal and kaolin catalyzed process at optimum conditions of catalyst-to-plastic ratio of 1:3, temperature of 450 °C and 30 min were characterized to determine their suitability for use as fuel. The liquid product from thermal cracking is highly viscous, dark brown in colour and contains wax, while that from catalytic pyrolysis is highly volatile and light yellow in colour. The difference in colour can be attributed to the use of catalyst which facilitated the secondary cracking of the plastic to lighter products. The results prove that the use of catalyst in pyrolysis of polypropylene helps to improve quality and increase yield. Similar oil colour (brownish yellow) was observed by Sonawane et al. [8] when 10% NZ catalyst was used in the pyrolysis of polypropylene.
Density is the weight occupied by a substance per unit volume; it is a property which determines the injection and ignition quality of a fuel [24]. The density of the thermal cracking liquid product obtained in this study is 1.060 g/ml, while that of kaolin catalytic cracking process is 0.800 g/ml. Thus, the density of the liquid product from thermal cracking process is higher than that of the catalytic process. This is expected as the liquid product obtained from kaolin catalytic cracking is light and wax free. On comparing with gasoline with a reported density range of 0.72–0.78 g/ml and diesel oil with density range of 0.82–0.90 g/ml, the kaolin catalytic liquid product density is between the reported density ranges of gasoline and diesel. The thermal cracking oil product density is absolutely out of gasoline and diesel oil density ranges as it is heavier than both of them. The two liquid product densities from these studies indicate that there are more breaking of the polypropylene C–C covalent bonds to a remarkable extent during the catalytic cracking than during thermal cracking as secondary cracking is facilitated by the use of kaolin as a catalyst. The density of the catalytic pyrolytic liquid obtained is comparable to that of other pyrolytic oils reported: HDPE pyrolytic oil has 0.801 g/ml as density [25], LDPE pyrolytic oil was reported to have 0.815 g/ml as density [9], and PP pyrolytic oil has density of 0.778 g/ml as reported by Panda and Singh [16]. The very little variation can be due to differences in pyrolysis condition, catalyst used, differences in polymeric material, and differences in the method of evaluation. The density of the liquid product obtained from kaolin catalyzed pyrolysis in this study suggests that it can be suitable for use as fuel, while that of thermal cracking might not be suitable.
Viscosity is an important property which measures the resistance of fluid flow which is related directly to pressure, temperature, and film formation [17]. Viscosity is the most crucial property of any lubricating oil or fuel oil which signifies how well a fuel will flow in automobile engines [26]. A high viscous fluid is not good for automobile engines as it can cause mechanical inefficiency, also a low viscous fluid is not also recommended as the fuel will drain away faster in engines [27]. The viscosity of the catalytic pyrolysis liquid product obtained in this work is 2.72 cSt, while that of thermal pyrolysis is 4.37 cSt. The higher viscosity value of the liquid product from thermal cracking is due to its waxy nature. These viscosity values are within the viscosity value range of 2.0–4.5 cSt for diesel but higher than the viscosity range of 1.0–2.1 cSt for gasoline.
Different pyrolytic oils have been documented with viscosities similar to the one obtained in this study; 3.3 cSt was reported for HDPE pyrolytic oil by Kumar and Singh [25], 2.4 cSt was reported by Na et al. [9] for LDPE, and Panda and Singh [16] reported 2.27 cSt as the viscosity of PP pyrolytic oil. All the values reported were within similar range; however, the little discrepancies observed might be due to differences in methods of evaluation, differences in polymer feedstock cracked, and differences in pyrolysis condition.
Flash point is defined as the minimum temperature at which the vapour from a liquid is sufficient to ignite when exposed to flame [27]. Fire point is the temperature at which the vapour formed as a result of ignition continues to burn for at least 5 s. Flash point and fire point are significant at high temperatures. They indicate the maximum temperature at which fuel can be stored and handled without serious fire hazard [28]. Flash point and fire point of 40 and 47 °C, respectively, were obtained for the catalytic pyrolytic liquid, while 78 and 85 °C were obtained for the thermal pyrolytic liquid. It is expected that the catalytic pyrolytic liquid which is light, non-viscous and wax free should be more volatile and ignites in open flame at lower temperature than the thermally pyrolytic liquid which is viscous. The kaolin catalyzed pyrolytic liquid flash and fire points were within the flash and fire points range of 37–42 and 42–47 °C for gasoline, respectively, but considerably lower than that of diesel oil with flash point range of 55–80 °C and fire point range of 60–90 °C. However, the thermal pyrolytic liquid flash and fire points are remarkably out of gasoline flash and fire points range but within diesel oil flash and fire points’ range.
Flash point and fire point of the liquid products are in agreement with reported values for pyrolytic oils. Few works presented a significant difference in the flash point of pyrolytic oil; Na et al. [9] obtained 70 °C as flash point for HDPE oil; Panda [19] reported a flash point of 22 °C for PP oil. The notable difference can be attributed to variation in the methods of evaluation, modes of pyrolysis employed, and types of catalyst used. The result of this study indicates that the kaolin catalyzed pyrolytic liquid obtained has a low flash point and fire point and as such should be handled with care to avoid serious fire hazard. This low flash point confirms the suitability of the liquid product as fuel for automobile engines.
Furthermore, the pour point (which is a measure of fluid flow ability under colder operating temperature and conditions) of the catalytic pyrolytic liquid was determined to be − 18 °C, while that of thermal pyrolysis was − 5 °C. Thermal pyrolytic liquid with high viscosity and density is expected to have high pour point than the catalytic pyrolitic liquid, because secondary and absolute degradation of the C–C bond have been facilitated by the use of the kaolin catalyst [14]. The pour point of the catalytic pyrolytic liquid is close to that of gasoline (− 12 °C) but within the range of that of diesel oil (− 40 to − 15 °C). The pour point of the thermal pyrolytic liquid is higher than the pour point of both gasoline and diesel oil which implies that it is not suitable for use in cold temperate regions. The oil pour point is below zero, which is the recommended value for fuel [29].
Finally, the calorific value of the catalytic pyrolytic liquid is 46.479 MJ/k, while that of thermal pyrolytic liquid is 35.460 MJ/kg. Heating or energy or calorific value of a fuel refers to the amount of heat evolved during combustion of a given amount of it [27]. High calorific values indicate that such substance would release great amount of heat energy during complete combustion with air under standard conditions. For a substance to be suitable for use as fuel, it must have considerably high amount of heating value which would make it burn in the air for a very long time [29]. The calorific value (CV) of the kaolin catalytic liquid is remarkably higher than gasoline CV of 42.702 MJ/kg and that of diesel oil with CV of 45.814 MJ/kg. On the other hand, the CV of thermal pyrolytic liquid is significantly lower than the CVs of both gasoline and diesel oil. This implies that the catalytic pyrolytic liquid would function well as fuel in automobile engines. The heating values documented from various studies for pyrolytic oil were in close range to the one obtained in this work. Little variation exists in the work of Kumar and Singh [25] with 64.302 MJ/kg as PP calorific value. Difference in the type and grades of PP used could account for the observed disparity.
FTIR characterization of the liquid fuel
FTIR analysis of the liquid fuel at optimum condition was carried out to reveal the presence of different functional groups within the waveband of 400–4000 cm−1 with the highest signal occurring at 47%T showing different types of vibrations (Fig. 2). According to Coates [30], a broad band of 426.28–725.2 cm−1 indicates the presence of sulphates (S–S, C–S stretch vibration), the presence of which can be attributed to the sulphur present in the natural gas; propylene which was the monomer of the polymer; polypropylene. Short wave band 2729.37–3068.85 cm−1 denotes the presence of aromatic alkyl groups (C–H stretch); this is expected as the oil is obtained from degradation of polypropylene with a molecular formula of [–C3H5–]n which contains carbon and hydrogen [30]. Spectrum in the waveband of 3437.26–3848.12 cm−1 confirms the presence of hydroxyl groups (O–H, C–O–O–H stretch); the presence of hydroxyl groups can be largely attributed to the additives added during polymer processing [31]. It was reported by Panda and Singh [16] that polypropylene has higher affinity to oxidation than other polymeric materials because of the presence of tertiary carbon which is bonded to a methyl group and can easily form peroxide in the chemical form of (–C–O–O–H) in the presence of oxygen even at lower temperature of about 150 °C. Peroxides are further decomposed to a more stable oxygen containing compounds such as hydroxyl (–O–H), carbonyl (–C–O), and nitro (–N–O) groups. Other functional groups present were amine (C–N, N–H) with a waveband of 1159.26 and 1649.19 cm−1, aromatic rings (C–C double bond) of wavelength, 1454.38 cm−1 and organohalogens (C–Cl and C–Br) with a wavelength of 725.26 and 835.21 cm−1, respectively. The presence of impurities in the unmodified kaolin used might be a contributory factor to the presence of amine, phosphates, organohalogens functional groups. A significant variation exist in the FTIR spectrum of PP pyrolytic oil reported by Panda and Singh [16] which documented the presence of mainly olefins with few indication of esters, ethers, carbonyl, alcohols, and carboxylic acid groups. This significant variation can be largely due to the differences in the grade of kaolin and polypropylene used.
Conclusion
Polypropylene plastic waste is convertible into usable liquid fuel via pyrolysis technique at an optimum condition of 450 °C and at a catalyst-to-plastic ratio of 1:3. Ahoko kaolin was found to be effective as a low-cost catalyst for the degradation of PP to gasoline/diesel grade fuel. The local kaolin has selectivity for liquid product than solid and gaseous products of pyrolysis. The quality of the oil obtained from the kaolin catalyzed pyrolysis reaction is better than the uncatalyzed reaction. Thermal pyrolysis produced a highly viscous liquid fuel which is accompanied with the formation of wax, while the oil obtained from the catalytic pyrolysis was light, less viscous liquid without wax as kaolin facilitated secondary cracking of the oil into lighter products. Physio-chemical properties of the kaolin catalyzed pyrolytic oil confirmed the suitability of the PP liquid product as fuel with most properties comparable to those of conventional fuels with similarity peculiar to gasoline. Thermal pyrolytic liquid fuel needs further upgrade to be suitable for use as fuel. The FTIR results showed that the oil contains hydrocarbons with various functional groups.
References
Tursunov O (2014) A comparison of catalysts zeolite and calcined dolomite for gas production from pyrolysis of municipal solid waste (MSW). Ecol Eng 69:237–243
Bulushev DA, Ross JR (2011) Catalysis for conversion of biomass to fuels via pyrolysis and gasification: a review. Catal Today 171:1–13
Ates F, Norbert M, Nikolett B (2013) Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: product yields, gas and pyrolysis oil properties. Biores Technol 133:443–454
Khaing TK, Chaw SS (2015) Effects of various catalysts on fuel oil pyrolysis process of mixed plastic wastes. Int J Adv Eng Technol 8(5):794–802
UNEP (2010) Training module for the assesment of plastic waste. International Environmental Technology Centre, Osaka
Panda AK, Singh RK (2011) Catalytic performances of kaoline and silica alumina in the thermal degradation of polypropylene. J Fuel Chem Technol 39(3):198–202
Satyendra ST, Krishna KK, Suresh PS (2013) Low cost catalyst synthesized from coal fly-ash for regaining liquid fuel from HDPE and its kinetic analysis. Int J Chem Petrochem Technol (IJCPT) 3(2):31–40
Sonawane Y, Shindikar M, Khaladkar M (2015) Use of catalyst in pyrolysis of polypropylene waste into liquid fuel. Int Res J Environ Sci 4(7):24–28
Na J-G, Jeong B-H, Seong-Soo Kion SH (2006) Pyrolysis of low density polyethylene using sunthetic catalyst produced from fly ash. J Mater Cycles Waste Manag 8:126–132
**ao X, Le D, Li L, Meng XC, Morishita K, Takarada T (2010) Catalytic steam gasification of biomass in fluidized bed at low temperature: conversion from livestock manure compost to hydrogen-reach syngas. Biomass Bioenergy 34:1505–1512
Jung S, Cho M, Kang B, Kim J (2010) Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process Technol 91:277–284
Dimitris S, Achilias LA (2014) Recent advances in the chemical recycling of polymers (PP, PS, LDPE, HDPE, PVC, PC, Nylon, PMMA). Mater Recycl Trends Perspect 3:64
Weibing D, **g L, Larry LA (2014) Hydrocracking of waste plastics to clean liquid fuels. University of Utah, Department of Chemical and Fuels Engineering. Salt Lake City, UT 841 12: 3290 ME9
Panda AK, Singh RK (2013) Optimization of process parameters by Taguchi method: catalytic degradation of polypropylene to liquid fuel. Int J Multidiscip Curr Res 4:50–54
Kovo AS (2008) Development of zeolite X and Y from Ahoko Nigerian Kaolin. PhD first year report, Chemical Engineering Department, The University of Manchester, United Kingdom
Panda AK, Singh RK (2013) Experimental optimization of process for the thermocatalytic degradation of waste polypropylene to liquid fuel. J Adv Energy Eng 1(3):74–84
Gao F (2010) Pyrolysis of waste plastics into fuels. Canterbury Publisher, University of Canterbury, New Zealand
Sharma BK, Moser BR, Vermillion KE, Doll KM, Rajagopalan N (2014) Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. J Fuel Process 122:79–90
Panda AK (2011) Studies on process optimization for production of liquid fuels from waste plastics; a thesis submitted in partial fulfilment for the award of the degree of doctorate of philosophy in chemical engineering. Chemical Engineering Department, National Institute of Technology Rourkela, Rourkela
Mi-** J, Suek Joo C, Kyung-Seun Y, Changkook R, Sung Hoon P, Jong M, Jong-Ki J, Young-Kwon P, Seungdo K (2011) Copyrolysis of block polypropylene with waste wood chip. Korean J Chem Eng 28(2):497–501. https://doi.org/10.1007/s11814-010-0497-8
Rui X, Baosheng J, Hongcang Z, Zhao** Z, Mingyao Z (2006) Air gasification of polypropylene plastic waste in fluidized bed gasifier. Key Laboratory of Clean Coal Power Generation and Combustion Technology 9:778–786
Panda AK, Singh RK, Mishra DK (2010) Thermolysis of waste plastics to liquid fuel: a suitable method for plastic waste management and manufacture of value added products—a world prospective. Renew Sustain Energy Rev 14(1):233–248
Aroke UO, El-Nafty UA (2014) XRF, XRD and FTIR properties and characterization of HDTMA-Br surface modified Organo-Kaolinite clay. Int J Emerg Technol Adv Eng 4(4):817–825
Bilal S, Mohammed-Dabo IA, Nuhu M, Kasim SA, Almustapha I, Yamusa YA (2013) Production of biolubricant from Jatropha curcas seed oil. J Chem Eng Mater Sci 4(6):72–79
Kumar S, Singh R (2013) Thermolysis of high-density polyethylene to petroleum products. J Petrol Eng 2013:987568
Kumar S, Panda AK, Singh RK (2011) A review on tertiary recycling of high density polyethylene to fuel. Resour Conserv Recycl 55:893–910
Musa U, Mohammed IA, Sadiq MM, Aberuagba F, Olurinde AO, Obamina R (2015) Synthesis and characterization of trimethylolpropane-based biolubricants from castor oil. In: Proceedings of the 45th annual conference of NSCHE, Warri, Nigeria, 5–7 November 2015
Kuye A, Ahiekpor J, Achaw OW (2016) Fast pyrolysis of NigerFia Danta Hardwood (Nesogordomna papaverifera) and Obeche Softwood (Triplochiton scleroxylon) Sawdust. Petrol Technol Dev J 6(7):72–81
Ituen E, Ijioma C (2012) Fuel properties of palm oil biodiesel and its blends with diesel. J Mater Resour 7(1&2):38–48
Coates J (2000) In: Meyers R (ed) Interpretation of infrared spectra, a practical approach. John Wiley & Sons Ltd, Chichester
Sarker M, Rashid MM, Molla M, Rahman MS (2012) A new technology proposed to recycle waste plastics into hydrocarbon fuel in USA. Int J Energy Environ (IJEE) 3(5):23–34
Sumitava K, Dignagar HSJ (2015) Institution of Student of Science. http://www.quora.com How do you identify petrol diesel and kereosene if they are kept in three containers. Answered 09 Nov 2015
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hakeem, I.G., Aberuagba, F. & Musa, U. Catalytic pyrolysis of waste polypropylene using Ahoko kaolin from Nigeria. Appl Petrochem Res 8, 203–210 (2018). https://doi.org/10.1007/s13203-018-0207-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-018-0207-8