Abstract
Photocatalytic ceramic adsorbents were prepared from locally sourced kaolinite clay minerals for the removal of copper and cobalt ions from high concentration aqueous solutions. The minerals were treated with mild acid before modification using silver nanoparticles sources and titanium-oxide nanoparticles. Batch adsorption experiment was carried out on the targeted ions and the results were analyzed by Langmuir and Freundlich equation at different concentrations (100–1000 mg/l). As-received raw materials do not exhibit any adsorption capacity. However, the adsorption isotherms for modified kaolinite clay ceramic adsorbents could be fitted well by the Langmuir model for Cu2+ and Co2+ with correlation coefficient (R) of up to 0.99705. The highest and lowest monolayer coverage (q max) were 93.023 and 30.497 mg/g for Cu2+ and Co2+, respectively. The separation factor (R L ) was less than one (<1), indicating that the adsorption of metal ions on modified ceramic adsorbent is favorable. The highest adsorbent adsorption capacity (K f ) and intensity (n) constants obtained from Freundlich model are 14.401 (Cu2+ on KLN-T) and 6.057 (Co2+ on KLN-T).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollution of water by toxic wastes, biological materials and natural organic matter (NOM) continues to create an overwhelming environmental challenges. Hence, the treatment of contaminated wastes should be given special consideration before being released into the environment (Rio and Patrick 2012). Contamination of waters with heavy metals ions leachate from industries and urban garbage dumps is a major worry since most heavy metals such as copper, cobalt, chromium, lead, etc. are not biodegradable and as such they tend to build up in living organisms with a permanent toxic and carcinogenic effect thereby causing various health issues. Also, pollution of water can occur at both the source and at the point of use; this is very much dependent on the surface of contact of the water. This necessitates and it makes a sensible conclusion that remediation of water should be at the source and the point of use. Heavy metals removal from wastewater remains a vital health and environmental issue, and various procedures have been suggested namely, chemical precipitation, membrane filtration, ion exchange, coagulation, adsorption and electrocoagulation (Ali et al. 2012). Meanwhile, adsorption has been considered a reliable process that can be used to remediate a mixture of contaminants with low concentrations (Chermisinoff and Ellerbusch 1978). Several synthesized and naturally occurring materials (Khan et al. 2015; Karaoğlu et al. 2010; Vijayakumar et al. 2009; Oubagaranadin and Murthy 2010; Tao et al. 2010; Irani et al. 2011) have been employed as adsorbent in different forms. Synthetic materials appear to be a particularly viable and effective for the removal of heavy metals particularly in low concentrations (Shawabkeh 1998). The use of synthetic adsorbents is considered not suitable for develo** countries because of its high manufacturing cost. Studies have been carried out in recent times to improve the sorption capacity of natural clay adsorbents by chemical modification of their surface (Choi et al. 2012; Han et al. 2009; Jeon et al. 2009; Han et al. 2006; Zou et al. 2009). The highest considered concentration for heavy metal adsorption in aqueous solution has mostly been between 200 and 300 mg/l. However, in real-life situations involving environmental contamination by heavy metal, the concentration is often high. Improving the capability of adsorbents to remove high concentration heavy metal is crucial for the survival of the fauna and flora in the real environment. In an emergency situation of high concentration heavy metal effluent release from factories, power plants or mining sites, the usual trace or low concentration pollutant adsorbents may not be suitable. Therefore, it is imperative that adsorbents with wider adsorption spectrum are formulated to remove heavy metal pollutants at high concentrations (>500 ppm).
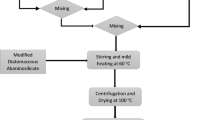
Kaolinite clay minerals are of particular importance as shown from recent examinations essentially due to their abundance, low cost and obtainability. The addition and elimination of metal ions using kaolinite clay is based on ion exchange and adsorption mechanisms, respectively. A number of studies about the use of kaolinite clay to remove heavy metal ions and basic dyes from aqueous solutions have been reported (Khan et al. 2012; Karaoğlu et al. 2010; Koyuncu et al. 2007). Nonetheless, most studies on kaolinite clay as adsorbent are based on removal of heavy metals at relatively low concentrations (≤300 mg/l) (Khan et al. 2015; Jiang et al. 2010; Bhattacharyya and Gupta 2006, 2008; Gupta and Bhattacharyyab 2008). Titanium oxide is a special chemical compound with numerous applications in micro-electronics. Likewise, photocatalytic nature of TiO2 has been exploited in environmental remediation studies (Hanaor et al. 2011; Hanaor and Sorrell 2014; Yang et al. 2012; Kujawa et al. 2013; Lasek et al. 2012; Kamegawa et al. 2013) for the removal of some hazardous minerals such as Cr(IV), nitric oxide, etc. Similarly, Wu et al. ( The kaolinite clay was collected from the exposed faces at a deposit site situated in Ijero Ekiti, Ekiti State, Nigeria. It is a soft, white clay mineral produced by the chemical weathering of aluminium silicate minerals like feldspar. Kaolinite clay is usually colored pink–orange–red by iron oxide, giving it distinct rust hue. Lighter concentrations of iron oxide yield white, yellow or light orange colors. The pure white color of clay may indicate little or no iron contamination which would have given it rusty color. All chemicals used in this work are reagents grade materials. The modifying materials are anatase TiO2 nanoparticle and AgNO3, which is the silver ion source. Sodium carbonate (99.0 %, Na2CO3) was used to reduce the silver ions. The Ag-TiO2 material expectedly served as a photocatalyst enhancing the remediation functionality of the main ceramic porous structure. Coir dust was the biomaterials incorporated into the ceramic adsorbent. Just like the geological minerals used, they are also abundant and low-cost organic material. The kaolinite clay mineral was carefully separated from the rock matrix and other materials by gently crushing the portion of interest in a mortar. A suspension of the pulverized material was prepared by adding the separated clay mineral particles to distilled water (1:5 clay to water ratio) in a 500 ml beaker; the kaolinite clay mineral totally immersed and soaked in the distilled water was left for about 2 h to aid dispersion of the clay particles in the water and for sol formation. Afterwards, the mixture was stirred for about 20 min and left undisturbed for about 2 min, enough for stone grains and other dense particles to settle and maintain the fine clay particles in suspension. The top milky sol containing the fine clay suspensions was poured into a separate beaker. This process was repeated until a clear kaolinite clay mineral separate was obtained. The white clay paste was dried for 18 h at 100 °C, forming a soft clay cake devoid of impurities. To beneficially enhance the physical and chemical properties of the kaolinite clay, mild acid treatment was carried out on the well-dried powder using 30 % dilute HNO3. The acid treatment was intended for dissolution of some amorphous materials in the raw materials. For photocatalytic activity of the adsorbents in the visible region, silver nanoparticles sources was loaded into the modifying anatase TiO2 nanopowder to give Ag-TiO2 (STOX). Silver nitrate (AgNO3) was used as the silver ions source. Firstly, 10 g of TiO2 powder was placed into a 500 ml beaker with 100 ml ethanol as the dispersion medium. Then, 0.1 M solution of AgNO3 and 1 % (w/v) solution of sodium carbonate (reducing agent) were prepared separately. Hence, 4.6 ml of the prepared silver nitrate solution and 5 ml of sodium carbonate were added into the beaker containing the dispersed TiO2. The mixture was stirred vigorously for 2 h using a magnetic stirrer to form a slurry solution and afterwards the solid material (Ag-TiO2) was collected by centrifugation. The collected modified TiO2 nanoparticle was dried in the furnace at 100 °C for about 24 h, thereafter; the powder was calcined at 400 °C for 12 h to remove leftover organics and also for thermal diffusion of the material. Ag-TiO2 modified kaolinite clay ceramic adsorbents were prepared using two main procedural routes. For the preparation of the ceramic adsorbent KLN-T, the prepared Ag-TiO2 powder (STOX) was ring milled with the acid-treated kaolinite aluminosilicate powder (KLN) containing coir dust. The milling was done to achieve adequate mechano-chemical interactions and even distribution of constituents in the ceramic composite. This was followed by solid state fusion, the milled mixture was subjected to a high temperature pressured treatment at 800 °C. To prepare ceramic adsorbent STOX-K, a dispersion of modified Ag-modified TiO2 was prepared as earlier described in “Kaolinitic aluminosilicate powder (KLN)”. Then, depending on the quantity proposed, 25 g of acid-treated kaolinite aluminosilicate powder mixed with a quantity of prepared coir dust was added slowly into the continuously stirred Ag-TiO2 colloidal solution. The slurry solution formed was stirred and mildly heated simultaneously for 2 h on the hot plate. The final modified kaolinite ceramic adsorbent STOX-K was recovered from the slurry solution via centrifugation. Noticeable visible light-induced color change was observed for the recovered STOX-K when exposed to daylight. The constituents of the ceramic precursors are highlighted in Table 1 (see Fig. 1). The stock solutions of Cu (II) and Co (II) were prepared from their commercial salts (nitrates and sulphates, respectively) and the solutions were standardized titrimetrically. Different required concentrations were prepared with appropriate dilutions from the stock. Batch adsorption tests were carried out in 50 ml Pyrex beakers. For each adsorption test, 0.125 g of raw and modified ceramic powder adsorbents were added to 20 ml of aqueous solutions at room temperature. The ion–adsorbent mixtures were shaken for 2 min for even distribution of the adsorbent particles in the solution and then kept for 12 h for saturation. The high concentration solutions compares to what is obtainable in real-life contamination scenarios. The aqueous phase was separated from the ceramic adsorbent by centrifugation after 12 h and the new equilibrium concentrations of the cation in final solution were monitored using UV–Visible spectrophotometer (Thermoscientific: Helio Omega). The proper absorbance-concentration calibration based on Beer-Lambert law was carried out at every stage of the spectrophotometric measurement to determine the equilibrium concentrations. The effect of initial concentrations of the aqueous solutions of Cu (II) and Co (II) on the adsorption capacity of the kaolinite ceramic adsorbents was also determined from the solutions with concentrations ranging from 100 to 1000 ppm. The amount adsorbed of Cu2+ and Co2+ (mg ion/g ceramic adsorbent) was calculated from the decrease in concentration in the media. The initial and equilibrium concentrations of the adsorbates were measured and compared to know the extent of the removal. The initial and final concentrations of the adsorbates are presented in Table 2. From the UV–Visible analysis of the initial and final metal solutions, necessary data were obtained and used in determining the adsorption isotherms. The amount of adsorbed Cu (II) and Co (II) ions (mg ions/g ceramic adsorbent) was calculated from the decrease in the concentrations of metal ions in the aqueous solutions using UV–Visible spectrophotometry, and by considering the adsorption volume and used mass of the kaolinite ceramic powder given by Eq. (1): where q
e
is the amount of metal ions adsorbed onto unit mass of the kaolinite clay powder (mg Cu2+ or Co2+/g adsorbent) at equilibrium; C
i
and C
e
are the concentrations of the metal ions in the initial solutions and in the aqueous phase after treatment for certain adsorption time; m is the amount of the ceramic powder used (g) and V is the volume of the adsorbate solution (liter). The effect of the initial adsorbate concentrations of the metal ions adsorbates was carried out in the study and the upper limit of the adsorption capacities of the kaolinite ceramic adsorbent for Cu2+ and Co2+ ions in solution were determined. The behavior of the ceramic adsorbent materials with respect to the concentration of the metal ions are shown in Figs. 2 and 3 for Cu2+ and Co2+, respectively. For Cu2+ adsorption, both samples STOX-K and KLN-T showed almost a linear relationship between the initial concentrations (mg/l) and quantity adsorbed (mg/g). While, for Co2+ adsorption, samples KLN-T and STOX-K showed distinct behavior as the initial concentration increased, with the highest Co2+ quantity adsorbed (35 mg/g) by STOX-K. It is observed that though quantity adsorbed rose with the concentrations, however, considering in percentage removal term, with the rise in the concentration, the percentage uptake by the samples steadily diminished. To understand the removal mechanism of heavy metals (Cu2+ and Co2+) by kaolinite clay ceramic adsorbent, it is essential to study the adsorption mechanisms of the modified materials, both in powder and pellets forms. It is acknowledged that ions and molecules, both in liquid and gaseous forms can adsorb on surface of certain adsorbents (Reed and Matsumoto 1993; Malik 2004), and certainly the likeliest mechanism that the prepared kaolinite ceramic removes metal ions from solutions is through the formation of monolayers on its surface by adsorbate particles. Adsorption is usually described through isotherms, that is, functions which connect the amount of adsorbate on the adsorbent. Distribution of metal ions between the liquid phase and the solid phase can be expressed by a number of isotherm models such as Langmuir and Freundlich. The Langmuir isotherm assumes monolayer adsorption onto a surface containing a finite number of adsorption sites of uniform strategies with no transmigration of adsorbate in the plane surface (Hameed et al. 2007). Once a site is occupied, no further sorption can take place at that site. This indicates that the surface reaches a saturation point where the maximum adsorption of the surface will be achieved. In this study, the adsorption of Cu (II) and Co (II) ions in solution was recorded in the concentration range from 100 to 1000 mg/l, mimicking a high Co2+ and Cu2+ contamination situations. The profiles obtained from the studies of the concentrations at room temperature were used to obtained Langmuir and Freundlich adsorption isotherms by using the well-known liquid isotherm equations. The linearized Langmuir isotherm is denoted by (2) The linear plots of specific adsorption (C
e
/q
e
) against the equilibrium concentrations (C
e
) shown in Fig. 4 showed that Cu2+ and Co2+ adsorptions on the kaolinite ceramic adsorbent obey the Langmuir model. The constants b and q
max have to do with the energy of adsorption and maximum adsorption capacity, and their values are obtained from the interception and slope of the plots, respectively, and are presented in Table 3. The Freundlich isotherm is introduced as an empirical model, where q
e
represents the amount of pollutant adsorbed per amount of adsorbent (ceramic) at the equilibrium (mg/g), C
e
represents the equilibrium concentration (mg/l), and K
f and n are parameters that depend on the adsorbate and adsorbent. Considering the Freundlich equation below: The equation can be linearized and the temperature dependent constants K
f
and 1/n found by linear regression as: where K
f
and n are Freundlich constants which correspond to adsorption capacity and adsorption intensity, respectively. Freundlich equilibrium constants were determined from the plot of ln q
e
versus ln C
e
from Fig. 5, on the basis of the linear form of Freundlich equation. The n value indicates the degree of nonlinearity between solution concentration and adsorption as follows: if n = 1, then adsorption is linear; if n < 1, then adsorption is a chemical process; if n > 1, then adsorption is a physical process. The n value obtained from Freundlich isotherm analyses for Cu2+ and Co2+ was found to be from 1.911 to 6.057 as shown in Table 3. The situation n > 1 is most common and may be due to a distribution of surface sites or any factor that causes a decrease in adsorbent–adsorbate interaction with increasing surface density (Reed and Matsumoto 1993) and the values of n within the range of 1–10 represent good adsorption (McKay et al. 1980; Ozer and Pirincci 2006). In the present study, since n lies between 1 and 7, it indicates the physical adsorption of metal ions onto kaolinite clay ceramic adsorbent. In both cases, linear plots were obtained, though the degree of linearity of the Langmuir isotherm plots is higher than that of Freundlich isotherms. However, this reveals the applicability of these isotherms on the ongoing adsorption process. Complete Langmuir and Freundlich constants derived from the isotherm plots are presented in Table 3 for the adsorption of metal ions on the adsorbent materials. Langmuir and Freundlich adsorption constants and correlation coefficients (R) are also shown in Table 3. To find the most appropriate model for the metal ions adsorption on the photocatalytic kaolinite adsorbents; data were fitted to Langmuir and Freundlich isotherm models and the results inferred that Langmuir adsorption isotherm was the best model for the metal ions adsorption on the modified kaolinite aluminosilicates with R of up to 0.99705 and 0.99635 in the case of Co2+ on STOX-K and Cu2+ on KLN-T, respectively. Significant features of Langmuir adsorption isotherm parameter can be used to predict or calculate the affinity between the sorbate and sorbent using a dimensionless constant called separation factor or equilibrium parameter (R
L
), which is articulated by the following relationship (Hall et al. 1966; Malik 2004): where b is the Langmuir constant and C
i
is the initial concentration. The value of R
L
indicated the type of Langmuir isotherm to be irreversible (R
L
= 0), linear (R
L
= 1), unfavorable (R
L
> 1), or favorable (0 < R
L
< 1) (McKay et al. 1982). The R
L
values between 0 and 1 indicate favorable adsorption. The R
L
value in the present investigation was found to be 0.012–0.242, showing that the adsorption of the metal ion the modified materials is favorable. Photocatalytic kaolinite ceramic adsorbents were prepared from silver nanoparticle source, titanium dioxide and kaolinite clay minerals for photo-degradation and removal of Cu2+ and Co2+ from high concentration aqueous solution. From the results obtained, it can be concluded that both photocatalytic kaolinite clay adsorbents are capable of removing Cu (II) and Co (II) ions from relatively high concentrated aqueous solutions. However, the adsorption capacity of adsorbent STOX-K is much higher than thermally fused KLN-T for the two metal ions. Freundlich and Langmuir models can be used to fit the data and estimate model parameters, but the overall data is slightly better fitted by Langmuir isotherm. Based on its availability and abundance, photocatalytic kaolinite clay ceramic adsorbent is environmentally friendly and economically viable for develo** countries. This ceramic adsorbent can be utilized as a pretreatment for high concentrated heavy metal effluents.Materials and methods
Raw materials
Kaolinitic aluminosilicate powder (KLN)
Modified kaolinite clay ceramic adsorbent (KLN-T and STOX-K)
Adsorption experiments
Results and discussions
Effect of initial concentrations of adsorbate
Cu2+ and Co2+ adsorption isotherm
Conclusions
References
Ali I, Khan TA, Mohd A (2012) Removal of arsenate from groundwater by electrocoagulation method. Environ Sci Pollut Res 19:1668–1676
Bhattacharyya KG, Gupta SS (2006) Kaolinite, montmorillonite, and their modified derivatives as adsorbents for removal of Cu(II) from aqueous solution. Sep Purif Technol 50(3):388–397
Bhattacharyya KG, Gupta SS (2008) Kaolinite and montmorillonite as adsorbents for Fe(III), Co(II) and Ni(II) in aqueous medium. Appl Clay Sci 41:1–9
Chermisinoff P, Ellerbusch F (1978) Carbon adsorption handbook. Ann Arbor Science, Ann Arbor
Choi J-W, Hong S-W, Kim D-J, Lee S-H (2012) October). Investigation of phosphate removal using sulphate-coated zeolite for ion exchange. Environ Technol 33(20):2329–2335
Gupta SS, Bhattacharyyab KG (2008) Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J Environ Manage 87:46–58
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5(2):212–223
Hameed BH, Din AT, Ahmad AL (2007) Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies. J Hazard Mater 141(3):819–825
Han R, Zhou W, Li H, Li Y, Shi J (2006) Copper (II) and Lead (II) removal from aqueous solution in fixed-bed columns by manganese oxide coated zeolite. J Hazard Mater 137:934–942
Han R, Zou L, Zhao X, Xu Y, Xu F, Li Y, Wang Y (2009) Characterization and properties of ion oxide-coated zeolite as adsorbent for removal of copper (II) from solution in fixed bed column. Chem Eng J 149:123–131
Hanaor DA, Sorrell CC (2014) Sand supported mixed-phase TiO2 photocatalysts for water decontamination applications. Adv Eng Mater 16(2):248–254
Hanaor D, Michelazzi M, Leonelli C, Sorrell CC (2011) The effects of firing conditions on the properties of electrophoretically deposited titanium dioxide films on graphite substrates. J Eur Ceram Soc 31(15):2877–2885
Irani M, Amjadi M, Mousavian MA (2011) Comparative study of lead sorption onto natural perlite, dolomite and diatomite. Chem Eng J 178:317–323
Jeon CS, Baek KT, Park JK, Oh YK, Lee SD (2009) Adsorption characteristics of As (V) on iron-coated zeolite. J Hazard Mater 163:804–808
Jiang M-Q, ** X-Y, Lu X-Q, Chen Z-L (2010) Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 252:33–39
Kamegawa T, Kido R, Yamahana D, Yamashita H (2013) Design of TiO2-zeolite composites with enhanced photocatalytic performances under irradiation of UV and visible light. Microporous Mesoporous Mater 165:142–147
Karaoğlu MH, Doğan M, Alkan M (2010) Kinetic analysis of reactive blue 221 adsorption on kaolinite. Desalination 256:154–165
Khan TA, Dahiya S, Ali I (2012) Use of kaolinite as adsorbent: equilibrium, dynamics and thermodynamic studies on the adsorption of rhodamine B from aqueous solution. Appl Clay Sci 69:58–66
Khan TA, Khan EA, Shahjahan (2015) Removal of basic dyes from aqueous solution by adsorption onto binary iron-manganese oxide coated kaolinite: non-linear isotherm and kinetics modeling. Appl Clay Sci 107:70–77
Koyuncu H, Kul AR, Yildiz N, Calimli A, Ceylan H (2007) Equilibrium and kinetic studies for the sorption of 3-methoxybenzaldehyde on activated kaolinites. J Hazard Mater 141:128–139
Kujawa J, Kujawski W, Koter S, Jarzynka K, Rozicka A, Bajda K, Larbot A (2013) Membrane distillation properties of TiO2 ceramic membranes modified by perfluoroalkylsilanes. Desalinat Water Treat 51(7–9):1352–1361
Lasek J, Yu Y-H, Wu JC (2012) Water and temperature effects on photo-selective catalytic reduction of nitric oxide on Pd-loaded TiO2 photocatalyst. Environ Technol 33(18):2133–2141
Malik PK (2004) Dye removal from wastewater using activated carbon developed from sawdust: adsorption equilibrium and kinetics. J Hazard Mater 113(1–3):81–88
McKay G, Otterburn MS, Sweeney AG (1980) The removal of colour from effluent using various adsorbents. III. Silica: rate processes. Water Res 14(1):15–20
McKay G, Blair HS, Gardner JR (1982) Adsorption of dyes on chitin. J Appl Polym Sci 27(8):3043–3057
Oubagaranadin JU, Murthy ZV (2010) Isotherm modeling and batch adsorber design for the adsorption of Cu(II) on a clay containing montmorillonite. Appl Clay Sci 50:409–413
Ozer A, Pirincci HB (2006) The adsorption of Cd(II) ions on sulphuric acid-treated wheat bran. J Hazard Mater 137(2):849–855
Reed BE, Matsumoto MR (1993) Modeling cadmium adsorption by activated carbon using the Langmuir and Freundlich isotherm expressions. Sep Sci Technol 28(13–14):2179–2195
Rio S, Patrick M (2012) Removal of metal ions from aqueous solution by adsorption onto low-cost biosorbent. Environ Technol 33(19):2211–2215
Shawabkeh R (1998) Synthesis of novel activated carbon from pecan shells and application to the adsorption of methylene blue, copper, and strontium from aqueous solutions. Ph.D. Dissertation, New Mexico State University, Las Cruces
Tao YF, Qiu Y, Fang SY, Liu ZY, Wang Y, Zhu JH (2010) Trap** the lead ion in multi-components aqueous solution by natural clinoptilolite. J Hazard Mater 180:282–288
Vijayakumar G, Dharmendirakumar M, Renganathan S, Sivanesan S, Baskar G, Elango KP (2009) Removal of congo red from aqueous solutions by perlite. Clean Soil Air Water 37:355–364
Wu P, **e R, Imlay JA, Shang JK (2009) Visible-light-induced photocatalytic inactivation of bacteria by composite photocatalysts of palladium oxide and nitrogen-doped titanium oxide. Appl Catal B 88(3–4):576–581
Yang JK, Lee SM, Farrokhi M, Giahi O, Shirzad Siboni M (2012) Photocatalytic removal of Cr(VI) with illuminated TiO2. Desalinat Water Treat 46(1–3):375–380
Zou W, Zhao L, Han R (2009) Removal of uranium (VI) by fixed-bed ion exchange column using natural zeolite coated with manganese oxide. Chin J Chem Eng 17(4):585–593
Acknowledgments
The support of Department of Physics and Centre for Energy Research and Development, Obafemi Awolowo University, Ile-Ife, Nigeria is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ajenifuja, E., Ajao, J.A. & Ajayi, E.O.B. Equilibrium adsorption isotherm studies of Cu (II) and Co (II) in high concentration aqueous solutions on Ag-TiO2-modified kaolinite ceramic adsorbents. Appl Water Sci 7, 2279–2286 (2017). https://doi.org/10.1007/s13201-016-0403-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0403-6