Abstract
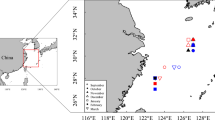
The migration route of oceanic squid provides critical information for us to understand their spatial and temporal variations. Mark-recapture and electronic tags tend to be problematic during processing. Cephalopod hard structures such as the beak, containing abundant ecological information with stable morphology and statolith-like sequences of growth increments, may provide information for studying spatio-temporal distribution. In this study, we developed a method, which is based on elemental concentration of beaks at different ontogenetic stages and sampling locations, to reconstruct the squid migration route. We applied this method to Ommastrephes bartramii in the North Pacific Ocean. Nine trace elements were detected in the rostrum sagittal sections (RSS) of the beak using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). For those elements, significant differences were found between the different ontogenetic stages for phosphorus (P), copper (Cu) and zinc (Zn). Sodium (Na), P and Zn were chosen as indicators of sea surface temperature (SST) and a regression model was estimated. The high probability of occurrence in a particular area represented the possible optimal squid location based on a Bayesian model. A reconstructed migration route in this study, combining all the locations at different ontogenetic stages, was consistent with that hypothesized in previous studies. This study demonstrates that the beak can provide useful information for identifying the migration routes of oceanic squid.
Similar content being viewed by others
References
Akaike H. 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6): 716–723, doi: https://doi.org/10.1109/TAC.1974.1100705
Alabia I D, Saitoh S I, Hirawake T, et al. 2016. Elucidating the potential squid habitat responses in the central North Pacific to the recent ENSO flavors. Hydrobiologia, 772(1): 215–227, doi: https://doi.org/10.1007/s10750-016-2662-5
Alabia I D, Saitoh S I, Mugo R, et al. 2015. Seasonal potential fishing ground prediction of neon flying squid (Ommastrephes bartramii) in the western and central North Pacific. Fisheries Oceanography, 24(2): 190–203, doi: https://doi.org/10.1111/fog.2015.24.issue-2
Arbuckle N S M, Wormuth J H. 2014. Trace elemental patterns in Humboldt squid statoliths from three geographic regions. Hydrobiologia, 725(1): 115–123, doi: https://doi.org/10.1007/s10750-013-1608-4
Arkhipkin A I. 2005. Statoliths as ‘black boxes’(life recorders) in squid. Marine and Freshwater Research, 56(5): 573–583, doi: https://doi.org/10.1071/MF04158
Arkhipkin A I, Shcherbich Z N. 2012. Thirty years’ progress in age determination of squid using statoliths. Journal of the Marine Biological Association of the United Kingdom, 92(6): 1389–1398, doi: https://doi.org/10.1017/S0025315411001585
Bettencourt V, Guerra A. 2000. Growth increments and biomineralization process in cephalopod statoliths. Journal of Experimental Marine Biology and Ecology, 248(2): 191–205, doi: https://doi.org/10.1016/S0022-0981(00)00161-1
Bower J R, Ichii T. 2005. The red flying squid (Ommastrephes bartramii): a review of recent research and the fishery in Japan. Fisheries Research, 76(1): 39–55, doi: https://doi.org/10.1016/j.fishres.2005.05.009
Boyle P, Rodhouse P G. 2005. Cephalopods. Ecology and Fisheries. Oxford: Blackwell Publishing, 222–233
Chen **njun, Liu Bilin, Chen Yong. 2008. A review of the development of Chinese distant-water squid jigging fisheries. Fisheries Research, 89(3): 211–221, doi: https://doi.org/10.1016/j.fishres.2007.10.012
Chen **njun, Lu Huajie, Liu Bilin, et al. 2012. Species identification of Ommastrephes bartramii, Dosidicus gigas, Sthenoteuthis oualaniensis and Illex argentines (Ommastrephidae) using beak morphological variables. Scientia Marina, 76(3): 473–481, doi: https://doi.org/10.3989/scimar.2012.76n3
Elsdon T, Gillanders B. 2005. Strontium incorporation into calcified structures: separating the effects of ambient water concentration and exposure time. Marine Ecology Progress Series, 285: 233–243, doi: https://doi.org/10.3354/meps285233
Fang Zhou, Liu Bilin, Chen **njun, et al. 2016a. Sexual asynchrony in the development of beak pigmentation for the neon flying squid Ommastrephes bartramii in the North Pacific Ocean. Fisheries Science, 82(5): 737–746, doi: https://doi.org/10.1007/s12562-016-1011-y
Fang Zhou, Li Jianhua, Thompson K, et al. 2016b. Age, growth, and population structure of the red flying squid (Ommastrephes bartramii) in the North Pacific Ocean, determined from beak microstructure. Fishery Bulletin, 114(1): 34–44, doi: 10.7755/FB
Fang Zhou, Thompson K, ** Yue, et al. 2016c. Preliminary analysis of beak stable isotopes (δ 13C and δ 15N) stock variation of neon flying squid, Ommastrephes bartramii, in the North Pacific Ocean. Fisheries Research, 177: 153–163, doi: https://doi.org/10.1016/j.fishres.2016.01.011
Franco-Santos R M, Vidal E A G. 2014. Beak development of early squid paralarvae (Cephalopoda: Teuthoidea) may reflect an adaptation to a specialized feeding mode. Hydrobiologia, 725(1): 85–103, doi: https://doi.org/10.1007/s10750-013-1715-2
Hernández-López J L, Castro-Hernández J L, Hernández-Garcia V. 2001. Age determined from the daily deposition of concentric rings on common octopus (Octopus vulgaris) beaks. Fishery Bulletin, 99(4): 679–684
Ichii T, Mahapatra K, Sakai M, et al. 2004. Differing body size between the autumn and the winter-spring cohorts of neon flying squid (Ommastrephes bartramii) related to the oceanographic regime in the North Pacific: a hypothesis. Fisheries Oceanography, 13(5): 295–309, doi: https://doi.org/10.1111/fog.2004.13.issue-5
Ichii T, Mahapatra K, Sakai M, et al. 2009. Life history of the neon flying squid: effect of the oceanographic regime in the North Pacific Ocean. Marine Ecology Progress Series, 378: 1–11, doi: https://doi.org/10.3354/meps07873
Igarashi H, Ichii T, Sakai M, et al. 2017. Possible link between interannual variation of neon flying squid (Ommastrephes bartramii) abundance in the North Pacific and the climate phase shift in 1998/1999. Progress in Oceanography, 150: 20–34, doi: https://doi.org/10.1016/j.pocean.2015.03.008
Ikeda Y, Arai N, Kidokoro H, et al. 2003. Strontium: calcium ratios in statoliths of Japanese common squid Todarodes pacificus (Cephalopoda: Ommastrephidae) as indicators of migratory behavior. Marine Ecology Progress Series, 251: 169–179, doi: https://doi.org/10.3354/meps251169
Ikeda Y, Arai N, Sakamoto W, et al. 1997. Comparison on trace elements in squid statoliths of different species’ origin: as available key for taxonomic and phylogenetic study. International Journal of PIXE, 7(3–4): 141–146
Ikeda Y, Yatsu A, Arai N, et al. 2002. Concentration of statolith trace elements in the jumbo flying squid during El Nino and non-El Niño years in the eastern Pacific. Journal of the Marine Biological Association of the UK, 82(5): 863–866, doi: https://doi.org/10.1017/S0025315402006264
Jennings S, Cogan S M. 2015. Nitrogen and carbon stable isotope variation in northeast Atlantic fishes and squids. Ecology, 96(9): 2568, doi: https://doi.org/10.1890/15-0299.1
Jereb P, Roper C F E. 2010. Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. In: Myopsid and Oegopsid Squids. Vol. 2. Rome: FAO Species Catalogue for Fishery Purposes, 269
Kato Y, Sakai M, Mmasujima M, et al. 2014. Effects of hydrographic conditions on the transport of neon flying squid Ommastrephes bartramii larvae in the North Pacific Ocean. Hidrobiológica, 24(1): 33–38
Liu Bilin, Cao Jie, Truesdell S B, et al. 2016. Reconstructing cephalopod migration with statolith elemental signatures: a case study using Dosidicus gigas. Fisheries Science, 82(3): 425–433, doi: https://doi.org/10.1007/s12562-016-0978-8
Liu Bilin, Chen **njun, Chen Yong, et al. 2013. Geographic variation in statolith trace elements of the Humboldt squid, Dosidicus gigas, in high seas of Eastern Pacific Ocean. Marine Biology, 160(11): 2853–2862, doi: https://doi.org/10.1007/s00227-013-2276-7
Liu Bilin, Chen Yong, Chen **njun. 2015a. Spatial difference in elemental signatures within early ontogenetic statolith for identifying Jumbo flying squid natal origins. Fisheries Oceanography, 24(4): 335–346, doi: https://doi.org/10.1111/fog.2015.24.issue-4
Liu Bilin, Chen **njun, Chen Yong, et al. 2015c. Determination of squid age using upper beak rostrum sections: technique improvement and comparison with the statolith. Marine Biology, 162(8): 1685–1693, doi: https://doi.org/10.1007/s00227-015-2702-0
Liu Bilin, Fang Zhou, Chen **njun, et al. 2015b. Spatial variations in beak structure to identify potentially geographic populations of Dosidicus gigas in the Eastern Pacific Ocean. Fisheries Research, 164: 185–192, doi: https://doi.org/10.1016/j.fishres.2014.12.001
Mereu M, Agus B, Cannas R, et al. 2015. Mark-recapture investigation on Octopus vulgaris specimens in an area of the central western Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom, 95(1): 131–138, doi: https://doi.org/10.1017/S002531541400112X
Miserez A, Li Youli, Waite J H, et al. 2007. Jumbo squid beaks: inspiration for design of robust organic composites. Acta Biomaterialia, 3(1): 139–149, doi: https://doi.org/10.1016/j.actbio.2006.09.004
Miserez A, Schneberk T, Sun Chengjun, et al. 2008. The transition from stiff to compliant materials in squid beaks. Science, 319(5871): 1816–1819, doi: https://doi.org/10.1126/science.1154117
Miserez A, Rubin D, Waite J H. 2010. Cross-linking chemistry of squid beak. Journal of Biological Chemistry, 285(49): 38115–38124, doi: https://doi.org/10.1074/jbc.M110.161174
Moltschaniwskyj N, Cappo M. 2009. Alternatives to sectioned otoliths: the use of other structures and chemical techniques to estimate age and growth for marine vertebrates and invertebrates. In: Green B S, Mapstone B D, Carlos G, et al, eds. Tropical Fish Otoliths: Information for Assessment, Management and Ecology. Dordrecht: Springer, 133–173
Murata M, Nakamura Y. 1998. Seasonal migration and diel vertical migration of the neon flying squid, Ommastrephes bartramii, in the North Pacific. In: Okutani T, ed. Contributed Papers to International Symposium on Large Pelagic Squids. Tokyo: Japan Marine Fishery Resources Research Center, 13–30
Navarro J, Coll M, Somes C, et al. 2013. Trophic niche of squids: Insights from isotopic data in marine systems worldwide. Deep Sea Research Part II: Topical Studies in Oceanography, 95: 93–102, doi: https://doi.org/10.1016/j.dsr2.2013.01.031
Nishikawa H, Igarashi H, Ishikawa Y, et al. 2014. Impact of paralarvae and juveniles feeding environment on the neon flying squid (Ommastrephes bartramii) winter-spring cohort stock. Fisheries Oceanography, 23(4): 289–303, doi: https://doi.org/10.1111/fog.2014.23.issue-4
Nishikawa H, Toyoda T, Masuda S, et al. 2015. Wind-induced stock variation of the neon flying squid (Ommastrephes bartramii) winter-spring cohort in the subtropical North Pacific Ocean. Fisheries Oceanography, 24(3): 229–241, doi: https://doi.org/10.1111/fog.12106
O’Dor R K, Balch N. 1985. Properties of iIlex illecebrosus egg masses potentially influencing larval oceanographic distribution. NAFO Science Council Studies, 9: 69–76
Perales-Raya C, Almansa E, Bartolomé A, et al. 2014b. Age validation in Octopus vulgaris beaks across the full ontogenetic range: beaks as recorders of life events in octopuses. Journal of Shellfish Research, 33(2): 481–493, doi: https://doi.org/10.2983/035.033.0217
Perales-Raya C, Bartolomé A, García-Santamaría M T, et al. 2010. Age estimation obtained from analysis of octopus (Octopus vulgaris Cuvier, 1797) beaks: Improvements and comparisons. Fisheries Research, 106(2): 171–176, doi: https://doi.org/10.1016/j.fishres.2010.05.003
Perales-Raya C, Jurado-Ruzafa A, Bartolomé A, et al. 2014a. Age of spent Octopus vulgaris and stress mark analysis using beaks of wild individuals. Hydrobiologia, 725(1): 105–114, doi: https://doi.org/10.1007/s10750-013-1602-x
R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Ripley B, Ribeiro P J, Diggle P J. 2001. Spatial Statistics in R and geoR: A Package for Geostatistical Analysis. Analysis, 6(1): 14–15
Rodríguez-Navarro A, Guerra A, Romanek C S, et al. 2006. Life history of the giant squid Architeuthis as revealed from stable isotope and trace elements signatures recorded in its beak. In: Moltschaniwskyj N, ed. Cephalopod Life Cycle. Cephalopod International Advisory Council Symposium 2006 (CIAC’ 06). 6–10 February 2006, Hotel Grand Chancellor, Hobart, Tasmania, 97
Semmens J M, Pecl G T, Gillanders B M, et al. 2007. Approaches to resolving cephalopod movement and migration patterns. Reviews in Fish Biology and Fisheries, 17(2–3): 401–423, doi: https://doi.org/10.1007/s11160-007-9048-8
Sims D W, Genner M J, Southward A J, et al. 2001. Timing of squid migration reflects North Atlantic climate variability. Proceedings of the Royal Society B: Biological Sciences, 268(1485): 2607–2611, doi: https://doi.org/10.1098/rspb.2001.1847
Slominski A, Tobin D J, Shibahara S, et al. 2004. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiological Reviews, 84(4): 1155–1228, doi: https://doi.org/10.1152/physrev.00044.2003
Staaf D J, Coop S C, Haddock S H, et al. 2008. Natural egg mass deposition by the Humboldt squid (Dosidicus gigas) in the Gulf of California and characteristics of hatchlings and paralarvae. Journal of the Marine Biological Association of the UK, 88(4): 759–770
Swan G A. 1974. Structure, chemistry, and biosynthesis of the melanins. In: Herz W, Grisebach H, Kirby G W, eds. Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. Vienna: Springer, 521–582
Tanaka H. 2001. Tracking the neon flying squid by the biotelemetry system, in the central North Pacific Ocean. Aquabiology (in Japanese), 23(6): 533–539
Tian Siquan, Chen **njun, Chen Yong, et al. 2009. Evaluating habitat suitability indices derived from CPUE and fishing effort data for Ommatrephes bratramii in the Northwestern Pacific Ocean. Fisheries Research, 95(2–3): 181–188, doi: https://doi.org/10.1016/j.fishres.2008.08.012
Tian Yongjun, Nashida K, Sakaji H. 2013. Synchrony in the abundance trend of spear squid Loligo bleekeri in the Japan Sea and the Pacific Ocean with special reference to the latitudinal differences in response to the climate regime shift. ICES Journal of Marine Science, 70(5): 968–979, doi: https://doi.org/10.1093/icesjms/fst015
VanBogelen R A, Olson E R, Wanner B L, et al. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. Journal of Bacteriology, 178(15): 4344–4366, doi: https://doi.org/10.1128/jb.178.15.4344-4366.1996
Venables W N, Ripley B D. 2002. Modern Applied Statistics with S. 4th ed. New York: Springer
Vijai D, Sakai M, Wakabayashi T, et al. 2015. Effects of temperature on embryonic development and paralarval behavior of the neon flying squid Ommastrephes bartramii. Marine Ecology Progress Series, 529: 145–158, doi: https://doi.org/10.3354/meps11286
Watanabe H, Kubodera T, Ichii T, et al. 2004. Feeding habits of neon flying squid Ommastrephes bartramii in the transitional region of the central North Pacific. Marine Ecology Progress Series, 266: 173–184, doi: https://doi.org/10.3354/meps266173
Watanabe H, Kubodera T, Ichii T, et al. 2008. Diet and sexual maturation of the neon flying squid Ommastrephes bartramii during autumn and spring in the Kuroshio-Oyashio transition region. Journal of the Marine Biological Association of the United Kingdom, 88: 381–389
Wearmouth V J, Durkin O C, Bloor I S M, et al. 2013. A method for long-term electronic tagging and tracking of juvenile and adult European common cuttlefish Sepia officinalis. Journal of Experimental Marine Biology and Ecology, 447: 149–155, doi: https://doi.org/10.1016/j.jembe.2013.02.023
Young R E, Harman R F. 1988. “Larva”, “paralarva” and “subadult” in cephalopod terminology. Malacologia, 29(1): 201–207
Zumholz K. 2005. The influence of environmental factors on the micro-chemical composition of cephalopod statoliths [dissertation]. Kiel: University of Kiel
Acknowledgements
The support of the scientific surveys by commercial jigging vessel F/V **hai 827 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: The National Natural Science Foundation of China under contract No. NSFC4147129; the China Postdoctoral Science Foundation under contract No. 2017M610277; the Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources (Shanghai Ocean University), Ministry of Education under contract No. A1-0203-00-2009-6; the Fund of Key Laboratory of Open-Sea Fishery Development, Ministry of Agriculture, China under contract LOF 2018-02.
Rights and permissions
About this article
Cite this article
Fang, Z., Liu, B., Chen, X. et al. Ontogenetic difference of beak elemental concentration and its possible application in migration reconstruction for Ommastrephes bartramii in the North Pacific Ocean. Acta Oceanol. Sin. 38, 43–52 (2019). https://doi.org/10.1007/s13131-019-1431-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-019-1431-5