Abstract
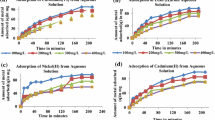
Laboratory-scale-simulated experiments were carried out using Cr(III) solutions to identify the Cr(III) retention behavior of natural red earth (NRE), a natural soil available in the northwestern coastal belt of Sri Lanka. The effects of solution pH, initial Cr(III) concentration and the contact time were examined. The NRE showed almost 100 % Cr(III) adsorption within the first 90 min. [initial [Cr(III)] = 0.0092–0.192 mM; initial pH 4.0–9.0]. At pH 2 (298 K), when particle size ranged from 125 to 180 μm the Cr(III) adsorption data were modeled according to Langmuir convention assuming site homogeneity. The pH-dependent Cr(III) adsorption data were quantified by diffused layer model assuming following reaction stoichiometries:
The present data showed that NRE can effectively be used to mitigate Cr(III) from aqueous solutions and this method is found to be simple, effective, economical and environmentally benign.




Similar content being viewed by others
References
Anderson MA, Ferguson JF, Gavis J (1976) Arsenate adsorption on amorphous aluminum hydroxide. Colloid Interface Sci 54(3):391–399
Calder LM (1988) Chromium contamination of groundwater. Adv Environ Sci Technol 20:215–229
Dahanayake K, Jayawardana SK (1979) Study of red and brown earth deposits of north-west Sri Lanka. J Geol Soc India 20:433–440
Demirbasa E, Kobyab M, Senturkb E, Ozkana T (2004) Adsorption kinetics for the removal of chromium(VI) from aqueous solutions on the activated carbons prepared from agricultural wastes. Water SA 30(4):533–539
Enviornmental Protection Agency (EPA) (1990) Environmental pollution control alternatives: EPA/625/5-90/025, EPA/625/4-89/023. EPA, Cincinnati
Harrison RM (2007) Principles of Environmental Chemistry.The Royal Society of Chemistry, GB, pp 383
Herbelin AL, Westall JC (1999) FITEQL Ver 4.0: a computer program for determination of equilibrium constant from experimental data. Report 99–01. Department of Chemistry, Oregon State University, Corvallis
Kim SD, Park KS, Gu MB (2002) Toxicity of hexavalent chromium to Daphnia magma: influence of reduction reaction by ferrous iron. J Hazard Mater 93(2):155–164
Lotfi M, Adhoum N (2002) Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep Purif Technol 26(2–3):137–146
Lu A, Zhong S, Chen J, Shi J, Tang J, Lu X (2006) Removal of Cr(VI) and Cr(III) from aqueous solutions and industrial wastewaters by natural clino-pyrrhotite. Environ Sci Technol 40(9):3064–3069
Malek NANN, Yusof AM (2007) Removal of Cr(III) from aquatic solutions using Zeolite: NaY repaired from rice husk ash. Malays J Anal Sci 11(1):76–83
Mauri R, Shinnar R, Amore MD, Giordano P, Volpe A (2001) Solvent extraction of chromium and cadmium from contaminated soils. Am Inst Chem Eng J 47(2):509–512
Modrogan C, Costache C, Orbulet DO (2007) Removal of hexavalent chromium from aqueous solutions by adsorption on peach kernel and nutshell. In: International proficiency testing conference, Sinaia, Romania. pp 371–377
Namasivayam C, Ranganathan K (1993) Waste Fe(III)/Cr(III) hydroxide adsorbent for the removal of Cr(VI) from aqueous solution and chromium plating industry wastewater. Environ Pollut 82(3):255–261
Ozer A, Altundogan HS, Erdem M, Tunmen F (1997) A study on the Cr(VI) removal from aqueous solutions by steel wool. Environ Pollut 97(1–2):107–112
Padilla A, Tavani EL (1999) Treatment of an industrial effluent by reverse osmosis. Desalination 129(1–3):219–226
Palmer CD, Puls RW (1994) Natural attenuation of hexavalent chromium in groundwater and soils. EPA Groundwater Issue: EPA154015-941505
Peris N (1998) Ability to purify contaminated water using natural red earth.Department of Geology University of Peradeniya, Sri Lanka, unpublished BSc thesis
Rengaraj S, Joo CK, Kim Y, Yi J (2003) Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H. J Hazard Mat 102:27–257
Srinivasan K, Balasubramanian N, Ramakrishna TV (1988) Studies on chromium removal by rice husk carbon. Indian J Environ Health 30:376–387
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley, New York
Tan WT, Ooi ST, Lee CK (1993) Removal of Cr(VI) from solution by coconut husk and palm pressed fibers. Environ Technol 14(3):277–282
Vithanage M, Chandrajith R, Bandara A, Weerasooriya R (2005) Mechanistic modeling of arsenic retention on natural red earth in simulated environmental system. J Colloid Interface Sci 294:265–272
Vithanage M, Senevirathna W, Chandrajith R, Weerasooriya R (2007) Arsenic binding mechanisms on natural red earth: a potential substrate for pollution control. Sci Total Environ 379(2–3):244–248
Weerasooriya R, Wijesekara HKDK, Bandara A (2001) Surface complexation modeling of cadmium adsorption on gibbsite. Colloids Surf 207(1–3):13–24
Zayed AM, Terry N (2003) Chromium in the environment: factors affecting biological remediation. Plant Soil 249:139–156
Zouboulis AI, Kydros KA, Matis KA (1995) Removal of hexavalent chromium anions from solutions by pyrite fines. Water Res 29:1755–1760
Acknowledgments
R.C. and C.B.D. gratefully acknowledged the Alexander von Humboldt (AvH) Foundation, Germany for the donation of a Varian 240FS Atomic Absorption Spectrophotometer used in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikagolla, C., Chandrajith, R., Weerasooriya, R. et al. Adsorption kinetics of chromium(III) removal from aqueous solutions using natural red earth. Environ Earth Sci 68, 641–645 (2013). https://doi.org/10.1007/s12665-012-1767-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1767-z