Abstract
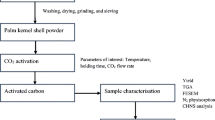
Date palm being one of the major biomass sources, largely being cultivated in UAE, is assessed for its potential to be an appropriate precursor for preparation of porous carbon. Porous carbon is prepared using the date palm pits in a tubular furnace with CO2 as the activating agent assessing the effect of process variables activation temperature, activation time and CO2 flow rate. Process optimization is performed to identify the process conditions that maximize the yield and BET surface area of the porous carbon using surface response methodology (RSM) based on central composite design (CCD). The influence of process parameters on the properties of porous carbon is investigated using analysis of variance (ANOVA) to identify the significant parameters. The optimum conditions for the preparation of porous carbon has been identified to be an activation temperature of 971°C, activation time of 56 min and CO2 flow rate of 5.1 cc/min based on the maximizing the yield and BET surface area. The optimum yield and BET surface area has been predicted to be 14.8% and 666 m2/g using the process optimization software. The textural characteristic of the optimized sample using nitrogen adsorption isotherm show the carbon to be predominantly microporous with the mean pore diameter estimated to be 1.76 nm using NLDFT method.










Similar content being viewed by others
References
Mohan, D., Singh, K.P., Singh, V.K.: Wastewater treatment using low cost activated carbons derived from agricultural byproducts—a case study. J. Hazard. Mater. 152, 1045–1053 (2008)
Singh, K.P., Malik, A., Sinha, S., Ojha, P.: Liquid-phase adsorption of phenols using activated carbons derived from agricultural waste material. J. Hazard. Mater. 150, 626–641 (2008)
Tan, I.A.W., Ahmad, A.L., Hameed, B.H.: Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 154, 337–346 (2008)
Hameed, B.H., El-Khaiary, M.I.: Sorption kinetics and isotherm studies of a cationic dye using agricultural waste: broad bean peels. J. Hazard. Mater. 154, 639–648 (2008)
Sahu, J.N., Agarwal, S., Meikap, B.C., Biswas, M.N.: Performance of a modified multi-stage bubble column reactor for lead(II) and biological oxygen demand removal from wastewater using activated rice husk. J. Hazard. Mater. 161, 317–324 (2009)
Demirbas, E., Kobya, M., Konukman, A.E.S.: Error analysis of equilibrium studies for the almond shell activated carbon adsorption of Cr(VI) from aqueous solutions. J. Hazard. Mater. 154, 787–794 (2008)
Karagozoglu, B., Tasdemir, M., Demirbas, E., Kobya, M.: The adsorption of basic dye (Astrazon Blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell activated carbon: Kinetic and equilibrium studies. J. Hazard. Mater. 147, 297–306 (2007)
Bota, A., Laszlo, K., Nagy, L., Lajos, G., Copitzky, T.: Comparative study of active carbons from different precursors. Langmuir 13, 6502–6509 (1997)
El-Naas, M.H., Al-Zuhair, S., Abu Alhaija, M.: Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J. Hazard. Mater. 173, 750–757 (2010)
Salman, J.M., Njoku, V.O., Hameed, B.H.: Bentazon and carbofuran adsorption onto date seed activated carbon: kinetics and equilibrium. Chem. Eng. J. 173, 361–368 (2011)
Hameed, B.H., Salman, J.M., Ahmad, A.L.: Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J. Hazard. Mater. 163, 121–126 (2009)
Al-Baker, A.: Date Palm. Al-Any Press, Baghdad (1972)
Alam, Z., Muyibi, S.A., Toramae, J.: Statistical optimization of adsorption processes for removal of 2,4-dichlorophenol by activated carbon derived from oil palm empty fruit bunches. J. Environ. Sci. 19, 674–677 (2007)
Karacan, F., Ozden, U., Karacan, S.: Optimization of manufacturing conditions for activated carbon from Turkish lignite by chemical activation using response surface methodology. Appl. Therm. Eng. 27, 1212–1218 (2007)
Bacaoui, A., Yaacoubi, A., Dahbi, A., Bennouna, C., Phan Tan Luu, R., Maldonado Hodar, F.J., Rivera-utrilla, J., Moreno-Castilla, C.: Optimization of conditions for the preparation of activated carbons from olive-waste cakes. Carbon 39, 425–432 (2001)
Azargohar, R., Dalai, A.K.: Production of activated carbon from Luscar char: experimental and modeling studies. Microporous. Mesoporous. Mater. 85, 219–225 (2005)
**n-hui, D., Srinivasakannan, C., **-hui, P., Li-bo, Z., Zheng-yong, Z.: Preparation of activated carbon from Jatropha hull with microwave heating: optimization using response surface methodology. Fuel. Process. Technol. 92, 394–400 (2011)
**e, Q.: Study on Control Over Coal Carbonization and Preparation of Activation Carbon. China Univ. of Mining & Tech, Xuzhou (1996)
Lyubchik, S.B., Benoit, R., Beguin, F.: Influence of chemical modification of anthracite on the porosity of the resulting activated carbons. Carbon 40, 287–294 (2002)
Bisio, C., Gatti, G., Boccaleri, E., Marchese, L., Superti, G.B., Pastore, H.O., Thommes, M.: Understanding physico-chemical properties of saponite synthetic clays. Microporous. Mesoporous. Mater. 90, 107–112 (2008)
Lastoskie, C., Gubbins, K.E., Quirke, N.: Pore size distribution analysis and net working: studies of microporous sorbents, in characterization of porous solids. Stud. Surf. Sci. Catal. 87, 51–61 (1994)
Acknowledgments
The Authors wish to acknowledge the financial support from The petroleum Institute for giving an opportunity to work on Activated carbon for gas processing research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, K.S.K., Al Shoaibi, A. & Srinivasakannan, C. Activated Carbon from Date Palm Seed: Process Optimization Using Response Surface Methodology. Waste Biomass Valor 3, 149–156 (2012). https://doi.org/10.1007/s12649-011-9104-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-011-9104-4