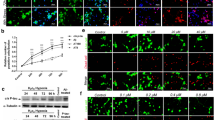
Abstract
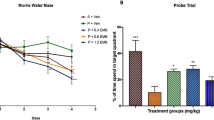
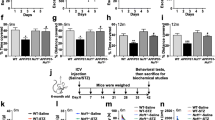
Alzheimer’s disease (AD) is the most common cause of progressive decline of memory function in aged humans. To study about a disease mechanism and progression, animal models for the specific disease are needed. For AD, although highly valid animal models exist, none of the existing models recapitulates all aspects of human AD. The pathogenic mechanisms involved in AD are diverse and thus it is difficult to recapitulate human AD in model organisms. Intracerebroventricular (ICV) injection of okadaic acid (OKA), a protein phosphatase 2A (PP2A) inhibitor, in rats causes neurotoxicity associated with neurofibrillary degeneration. However, this model lacks amyloid pathology as observed in AD. We aimed at combining two different treatments and hence producing a better animal model of AD which may mimic most of the neuropathological, neurobehavioral, and neurochemical changes observed in AD. For this, OKA (200 ng) was microinjected bilaterally into the hippocampus of male Wistar rats followed by exposure of same rats to hypoxic conditions (10%) for 3 days. The result of which, the combination model exhibited tau hyperphosphorylation along with Aβ upregulation as evident by western blotting and immunohistochemistry. The observed changes were accompanied with dysfunction of neurotransmitter system, i.e., decreased acetylcholine activity and expression. This combinatorial model also exhibited cognitive deficiency which was assessed by Morris water maze and avoidance tests along with enhanced oxidative stress which is thought to be a major player in AD pathogenesis. Taken together, we established an easily reproducible and reliable rat model for sporadic dementia of Alzheimer’s type in rats which allows effective testing of new therapeutic strategies.






Similar content being viewed by others
Abbreviations
- Aβ:
-
Beta amyloid
- NFTs:
-
Neurofibrillary tangles
- OKA:
-
Okadaic acid
- ROS:
-
Reactive oxygen species
- PHF:
-
Paired helical filament
- H2-DCFHDA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- SOD:
-
Superoxide dismutase
References
Alzheimer’s Association (2015) 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11:332–384
Alzheimer’s Association (2016) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12:459–509
Anand R, Kaushal A, Wani WY, Gill KD (2012) Road to Alzheimer’s disease: the pathomechanism underlying. Pathobiology 79:55–71
Areosa SA, Sherriff F (2003) Memantine for dementia Cochrane Database Syst Rev:CD003154
Braak H, Braak E (1996) Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl 165:3–12
Broetto N, Hansen F, Brolese G, Batassini C, Lirio F, Galland F, dos Santos JPA, Dutra MF, Gonçalves CA (2016) Intracerebroventricular administration of okadaic acid induces hippocampal glucose uptake dysfunction and tau phosphorylation. Brain Res Bull 124:136–143
Bromley-Brits K, Deng Y, Song W (2011) Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp 20:2920
Chavez JC, Agani F, Pichiule P, LaManna JC (2000) Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol (1985) 89:1937–1942
Choi PM, Zelig MP (1994) Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut 35:950–954
Cotman CW, Su JH (1996) Mechanisms of neuronal death in Alzheimer’s disease. Brain Pathol 6:493–506
Dimitrova DS, Getova-Spassova DP (2006) Effects of galantamine and donepezil on active and passive avoidance tests in rats with induced hypoxia. J Pharmacol Sci 101:199–204
Dwivedi S, Nagarajan R, Hanif K, Siddiqui HH, Nath C, Shukla R (2013) Standardized extract of Bacopa monniera attenuates okadaic acid induced memory dysfunction in rats: effect on Nrf2 pathway. Evid Based Complement Alternat Med 2013:294501
Forny-Germano L, Lyra e Silva NM, Batista AF, Brito-Moreira J, Gralle M, Boehnke SE, Coe BC, Lablans A, Marques SA, Martinez AMB, Klein WL, Houzel JC, Ferreira ST, Munoz DP, de Felice FG (2014) Alzheimer’s disease-like pathology induced by amyloid-beta oligomers in nonhuman primates. J Neurosci 34:13629–13643
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66:137–147
Gao L, Tian S, Gao H, Xu Y (2013) Hypoxia increases Abeta-induced tau phosphorylation by calpain and promotes behavioral consequences in AD transgenic mice. J Mol Neurosci 51:138–147
Glowinski J, Iversen L (1966) Regional studies of catecholamines in the rat brain. 3. Subcellullar distribution of endogenous and exogenous catecholamines in various brain regions. Biochem Pharmacol 15:977–987
Gordon RY, Mugantseva EA, Khutzian SS, Podolski IY (2009) Cycloheximide-induced inhibition of protein synthesis in hippocampal pyramidal neurons is time-dependent: differences between CA1 and CA3 areas. Neurosci Lett 461:249–251
Honig LS, Tang MX, Albert S, Costa R, Luchsinger J, Manly J, Stern Y, Mayeux R (2003) Stroke and the risk of Alzheimer disease. Arch Neurol 60:1707–1712
Kamat PK, Rai S, Nath C (2013a) Okadaic acid induced neurotoxicity: an emerging tool to study Alzheimer's disease pathology. Neurotoxicology 37:163–172
Kamat PK, Rai S, Swarnkar S, Shukla R, Ali S, Najmi AK, Nath C (2013b) Okadaic acid-induced Tau phosphorylation in rat brain: role of NMDA receptor. Neuroscience 238:97–113
Kamat PK, Rai S, Swarnkar S, Shukla R, Nath C (2014) Molecular and cellular mechanism of okadaic acid (OKA)-induced neurotoxicity: a novel tool for Alzheimer’s disease therapeutic application. Mol Neurobiol 50:852–865
Kar S, Slowikowski SPM, Westaway D, Mount HTJ (2004) Interactions between β-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatry Neurosci 29:427–441
Ladner CJ, Lee JM (1998) Pharmacological drug treatment of Alzheimer disease: the cholinergic hypothesis revisited. J Neuropathol Exp Neurol 57:719–731
Lecanu L, Greeson J, Papadopoulos V (2006) Beta-amyloid and oxidative stress jointly induce neuronal death, amyloid deposits, gliosis, and memory impairment in the rat brain. Pharmacology 76:19–33
Maddahi A, Edvinsson L (2008) Enhanced expressions of microvascular smooth muscle receptors after focal cerebral ischemia occur via the MAPK MEK/ERK pathway. BMC Neurosci 9:85
Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML (1998) Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol 150:40–44
Mecocci P, MacGarvey U, Beal MF (1994) Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol 36:747–751
Melov S (2002) Animal models of oxidative stress, aging, and therapeutic antioxidant interventions. Int J Biochem Cell Biol 34:1395–1400
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Muir JL, Page KJ, Sirinathsinghji DJ, Robbins TW, Everitt BJ (1993) Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory and attention. Behav Brain Res 57:123–131
Nakamura S, Murayama N, Noshita T, Annoura H, Ohno T (2001) Progressive brain dysfunction following intracerebroventricular infusion of beta(1-42)-amyloid peptide. Brain Res 912:128–136
Ng KM, Lau CF, Fung ML (2010) Melatonin reduces hippocampal beta-amyloid generation in rats exposed to chronic intermittent hypoxia. Brain Res 1354:163–171
Ogunshola OO, Antoniou X (2009) Contribution of hypoxia to Alzheimer’s disease: is HIF-1alpha a mediator of neurodegeneration? Cell Mol Life Sci 66:3555–3563
Olanow CW (1993) A radical hypothesis for neurodegeneration. Trends Neurosci 16:439–444
Palmer AM, Burns MA (1994) Selective increase in lipid peroxidation in the inferior temporal cortex in Alzheimer’s disease. Brain Res 645:338–342
Partridge RS, Monroe SM, Parks JK, Johnson K, Parker WD Jr, Eaton GR, Eaton SS (1994) Spin trap** of azidyl and hydroxyl radicals in azide-inhibited rat brain submitochondrial particles. Arch Biochem Biophys 310:210–217
Piala JJ, High JP, Hassert GL Jr, Burke JC, Craver BN (1959) Pharmacological and acute toxicological comparisons of triflupromazine and chlorpromazine. J Pharmacol Exp Ther 127:55–65
Pichiule P, LaManna JC (2002) Angiopoietin-2 and rat brain capillary remodeling during adaptation and deadaptation to prolonged mild hypoxia. J Appl Physiol (1985) 93:1131–1139
Pratico D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, FitzGerald GA (2000) Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol 48:809–812
Price DL, Sisodia SS (1998) Mutant genes in familial Alzheimer’s disease and transgenic models. Annu Rev Neurosci 21:479–505
Reist M, Marshall KA, Jenner P, Halliwell B (1998) Toxic effects of sulphite in combination with peroxynitrite on neuronal cells. J Neurochem 71:2431–2438
Richer MJ, Lavallee DJ, Shanina I, Horwitz MS (2009) Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS One 4:e4127
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189
Sharma DR, Sunkaria A, Bal A, Bhutia YD, Vijayaraghavan R, Flora SJ, Gill KD (2009) Neurobehavioral impairments, generation of oxidative stress and release of pro-apoptotic factors after chronic exposure to sulphur mustard in mouse brain. Toxicol Appl Pharmacol 240:208–218
Snyder B, Shell B, Cunningham JT, Cunningham RL (2017) Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep 5(9):e13258
Squire LR (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99:195–231
Sriram K, Pai KS, Boyd MR, Ravindranath V (1997) Evidence for generation of oxidative stress in brain by MPTP: in vitro and in vivo studies in mice. Brain Res 749:44–52
Udayabanu M, Kumaran D, Nair RU, Srinivas P, Bhagat N, Aneja R, Katyal A (2008) Nitric oxide associated with iNOS expression inhibits acetylcholinesterase activity and induces memory impairment during acute hypobaric hypoxia. Brain Res 1230:138–149
Unkruer B et al (2009) Cellular localization of Y-box binding protein 1 in brain tissue of rats, macaques, and humans. BMC Neurosci 10:28
Wasilewski M, Wojtczak L (2005) Effects of N-acylethanolamines on the respiratory chain and production of reactive oxygen species in heart mitochondria. FEBS Lett 579:4724–4728
Watson C, Paxinos G (2007) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Wills ED (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99:667–676
Zhang X, Le W (2010) Pathological role of hypoxia in Alzheimer’s disease. Exp Neurol 223:299–303
Zhang Z, Simpkins JW (2010) Okadaic acid induces tau phosphorylation in SH-SY5Y cells in an estrogen-preventable manner. Brain Res 1345:176–181
Zhang QG, Wang R, Khan M, Mahesh V, Brann DW (2008) Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci 28:8430–8441
Funding
This study was funded by Council of Scientific and Industrial Research (CSIR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No conflicts of interest are declared by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Okadaic acid and hypoxia induce plaque and tangle pathology in this rat model of AD
• Combination of okadaic acid and hypoxia produced enhanced oxidative stress and associated neurodegeneration in rat brain
• Okadaic acid and hypoxia exposure to rats mimic the neurobehavioral and neurochemical alterations as observed in AD.
Rights and permissions
About this article
Cite this article
Kaushal, A., Wani, W.Y., Bal, A. et al. Okadaic Acid and Hypoxia Induced Dementia Model of Alzheimer’s Type in Rats. Neurotox Res 35, 621–634 (2019). https://doi.org/10.1007/s12640-019-0005-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-0005-9