Abstract
Purpose
Despite the popularity of the erector spinae plane (ESP) block, both the mechanism of the block and the extent of injectate spread is unclear. This study used magnetic resonance imaging (MRI) to evaluate the spread of local anesthetic injectate following ESP blocks in six patients with pain.
Methods
Six patients received a left-sided ultrasound-guided ESP block at the T10 level. The injectate contained 29.7 mL of 0.25% bupivacaine and 0.3 mL of gadolinium in the first patient, with an additional 5 mL (50 mg) of triamcinolone in the subsequent five patients. Sensory block to pinprick and cold as well as pain score (with 0 indicating no pain and 10 being maximum pain) were assessed 20 and 30 min respectively following the ESP block. MRI was performed one hour after the block.
Result
The injectate spread into the intercostal space and neural foramina in all six patients, but the extent of cephalocaudal spread was variable, with a median [interquartile range] spread of 9 [5–11] and 3 [2–6] levels for the intercostal space and neural foramina, respectively. The injectate also spread extensively within the erector spinae muscles. Spread to the epidural space was seen in two patients. Sensory block was achieved in both ventral and dorsal dermatomes in all patients, though the extent was variable.
Conclusions
Our study showed that the ESP block injectate consistently spread to the erector spinae muscles, neural foramina, and intercostal space. It was associated with sensory changes and pain relief in the dorsal and ventral thoracic and abdominal walls. Nevertheless, the extent of spread to the neural foramina and intercostal space, and the sensory block itself, was highly variable.
Résumé
Objectif
Malgré la popularité du bloc plan des érecteurs du rachis (PER), le mécanisme du bloc et l’ampleur de la diffusion du produit injecté ne sont pas clairement connus. Cette étude a utilisé l’imagerie par résonance magnétique (IRM) pour évaluer la diffusion de l’anesthésique local injecté après des blocs du PER chez six patients présentant des douleurs.
Méthodes
Six patients ont reçu un bloc du PER guidé par échographie du côté gauche au niveau T10. Le produit injecté contenait 29,7 mL de bupivacaïne 0,25 % et 0,3 mL de gadolinium pour le premier patient avec un supplément de 5 mL (50 mg) de triamcinolone pour les cinq patients suivants. Le bloc sensitif au toucher/piquer et au froid, ainsi que le score de douleur (où 0 indique une absence de douleur et 10, une douleur maximum) ont été évalués respectivement 20 et 30 minutes après le bloc du PER. Une IRM a été réalisée une heure après le bloc.
Résultat
Le produit injecté a diffusé dans l’espace intercostal et les foramens intervertébraux chez les six patients, mais l’étendue de la diffusion céphalocaudale a été variable avec une diffusion médiane [plage interquartile] de 9 [5 à 11] niveaux pour les espaces intercostaux et 3 [2 à 6] niveaux pour les foramens intervertébraux. Le produit injecté a également largement diffusé dans les muscles érecteurs du rachis. Une diffusion vers l’espace épidural a été observée chez deux patients. Un bloc sensitif des dermatomes ventraux et dorsaux a été obtenu chez tous les patients, bien que son étendue ait été variable.
Conclusions
Notre étude a montré que le produit injecté dans un bloc du PER diffusait constamment dans les muscles érecteurs du rachis, les foramens intervertébraux et les espaces intercostaux. Il a été associé à des modifications sensorielles et à un soulagement de la douleur dans les parois thoraciques et abdominales, ventrales et dorsales. Néanmoins, l’étendue de la diffusion vers les foramens intervertébraux et les espaces intercostaux, ainsi que le bloc sensitif proprement dit ont été très variables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The erector spinae plane (ESP) block was first reported by our group in 2016 as a technique for treating thoracic neuropathic pain.1 Because the technique is simple and the target site is relatively remote from important structures (i.e., pleura, spinal cord, or paravertebral space), a plethora of interest in this block soon followed. At the time of this article’s writing, more than 200 articles on the ESP block, including 16 randomized-controlled trials, have been published.2,3 The applications of this block have rapidly expanded to address anesthetic and analgesic applications in the thoracic, lumbar, and even cervical areas.4,5,6,7 Despite the popularity of the block, its mechanism of action and how the local anesthetic (LA) injectate spreads is unclear.8 Thus far, five cadaveric studies examining the mechanism of ESP block have been published, and the evidence on the spread to the intercostal or paravertebral spaces has been conflicting.9,10,11,12,13
To better characterize LA spread of the ESP block, contrast injection assessed by conventional radiography or magnetic resonance imaging (MRI) and corresponding clinical evaluation in live subjects may offer advantages.8,14 Therefore, the purpose of this in vivo prospective study in a cohort of patients with chronic pelvic pain was to examine the pattern of LA spread using MRI one hour following the ESP block. We also correlated the sensory blockade and pain outcome with the MRI findings of injectate spread.
Method
Following institutional ethics approval (9 February 2019) from the Hospital de Clínicas, Faculty of Medicine, University of the Republic (Montevideo, Uruguay), patients with chronic abdomino-pelvic pain who were scheduled for an ESP block were recruited for this study.
Patients experiencing chronic abdomino-pelvic pain for longer than three months and who were refractory to pharmacological and surgical management were referred to our pain clinic to evaluate their suitability for interventional pain management. In our clinic, we offer ESP block for patients with chronic pelvic pain because we have had favourable clinical experience with this treatment.15 Patients already consenting to an ESP block were subsequently approached to participate in the present MRI spread evaluation study. Of the 20 patients initially screened, six consented to participate in the study. Prior to inclusion in the study, we informed patients about the nature of the procedures as well as the potential risks and benefits of the intervention. Three separate informed consents were obtained (as suggested by the ethics committee): one for the ESP block, another for MRI imaging with gadolinium, and a third for collection of clinical data for publication.
Exclusion criteria included allergy to LAs, claustrophobia, allergy to contrast media, presence of metal implants, body mass index (BMI) > 40 kg·m−2, history of renal failure, and insulin-dependent diabetes.
Ultrasound-guided ESP block
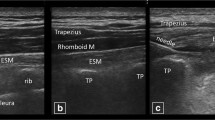
On the day of the study, the patient received the ESP in the block room in the hospital surgical area. After securing intravenous access and application of standard monitors, the patient was placed in the prone position. The ESP block that targeted the tip of the transverse process of the left T10 vertebra was performed by an experienced anesthesiologist (A.S.) as follows. Under sterile conditions, a linear (6–12 MHz) ultrasound (US) probe (VIVID, General Electric, Chino, CA, USA) was initially placed in a parasagittal plane 3–4 cm lateral to the spinous process over the last discernable rib (12th rib). The probe was then moved upwards to the tenth rib. From there, the US probe was rotated from parasagittal to transverse view to visualize the tip of the transverse process.16 The tip of the left T10 transverse process was then placed in the middle of the US screen and the US probe was again rotated to parasagittal view to visualize tip of the transverse process, which appeared as a flat distinguishable hyperechoic line.
Following skin infiltration with 3 mL of 2% lidocaine, a 22G 11-cm echogenic needle (SonoTap, Pajunk, Norcross, GA, USA) was inserted in-plane from cranial to caudal to the tip of the left T10 transverse process. Upon hydrolocation using normal saline with the erector spinae muscle lifting from the tip of transverse process, a mixture of 29.7 mL 0.25% bupivacaine and 0.3 mL gadolinium (Gadovist 1 mmol·mL−1; Bayer, Leverkusen, Germany) was administered in the first patient. In the subsequent five patients, an additional 5 mL of triamcinolone (50 mg) was added to the mixture, giving a total volume of 35 mL. The injection was slowly administered in aliquots of 5 mL. All patients underwent an MRI one hour after the procedure. Patients 2 and 3 also received an MRI before the ESP block.
Magnetic resonance imaging study
The MRI was performed using a 1.5 T long spine coil covering spine levels from T1 to L5 using a slice thickness of 3 mm. T1-weighted sequences with fat suppression were performed in sagittal, coronal, and axial planes.
The distribution pattern of the gadolinium-containing solution administered in the ESP plane was evaluated starting at T10 (where the ESP block was performed) with the axial T1-weighted fat suppression sequences analyzed in a cranial and caudal direction. The contrast was evaluated in the following structures: superficial (e.g., serratus posterior inferior and trapezius muscles) and erector spinae muscles in the paravertebral region, intercostal muscles (noting the maximum lateral spread in intercostal plane), neural foramina, and epidural space. As the neural foramen is part of the medial wall of the paravertebral space, contrast spreading to the neural foramen was considered to indicate a spread to the paravertebral space.
All images were reviewed and analyzed by the same neuroradiologist experienced in spine MRI images. Efforts were made to differentiate the contrast uptake from the various vascular structures. The contrast inside the vascular structures was recognized for the disposition characterized for the anatomic location and the elongated shape of the veins. The spread pattern of the contrast outside veins has a disorganized configuration not construed to the vascular structure and is in continuity with the site of injection.
Assessment of sensory block and analgesic effect
Abdomino-pelvic pain was assessed using an 11-point numeric rating scale (NRS; with 0 indicating no pain and 10 maximum pain) before and 30 min after the ESP block, as well as in subsequent follow-up visits. Twenty minutes following the ESP block, the dermatomal pattern of sensation to pinprick and cold (using ice) on the ipsilateral thoracoabdominal wall (dorsal and ventral surfaces) was assessed in the midclavicular line, xiphoid-pubic line, mid-axillary line, and interscapular midline. The sensation was graded as 0 (no sensation), 1 (decreased sensation), or 2 (normal). A sensory block of the dermatome was considered to be achieved if the block was either 0 or 1.
Descriptive statistics were used to present data using median [interquartile range (IQR)] as appropriate.
Results
Six female patients age between 34 and 50 yr with a BMI of 21–27 kg·m−2 were enrolled in this study. In all patients, the gadolinium-containing injectate spread extensively to the paraspinal muscles, intercostal space, and neural foramina (Fig. 1). The distribution of injectate in each patient is shown in Figs 2 and 3. Although the injectate spread consistently to the intercostal space and neural foramina in all six patients, the extent of spread was highly variable in the cephalocaudal levels. In the intercostal space, it spread to three levels in one patient, six levels in one patient, eight levels in one patient, and 11 levels in three patients (median [IQR] intercostal spread, 9 [5–11] levels). As for the neural foramina, contrast spread two levels in three patients, four levels in one patient, eight levels in one patient, and ten levels in one patient (median [IQR neural foramina spread, 3 [2–6] levels) (Fig. 2). The lateral spread to the intercostal space ranged from 5–8 cm from the midline; none of the spread was far enough to reach the angle of the rib. Spread to the epidural space was seen in only two patients (ten levels in one patient and two levels in another patient). The injectate spread extensively within the erector spinae muscles (multifidus and longissimus) and trapezius, mainly in the muscular planes, although intramuscular uptake was found at the site of injection (Fig. 3).
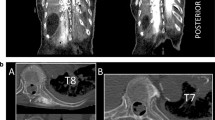
Magnetic resonance imaging (MRI) of the spine showing injectate spread. A) Sagittal MRI image showing that the spread of injectate can easily be seen with the addition of gadolinium MRI contrast (white line arrows). The injectate spread to the erector spinae muscles (*), paravertebral space (bold arrow), and neural foramina (arrowheads). The facet joint and the inferior articular process are indicated by a black arrow and white asterisk, respectively. B) Axial MRI view of T11 vertebra. The spread of injectate enhanced by gadolinium (line arrows) can be seen extended to the erector spinae muscle (black asterisks). The neural foramen is indicated by the arrowhead. DRG = dorsal root ganglion. The inferior articular process is indicated by a white asterisk. C) Sagittal MRI image. Epidural spread of the injectate is indicated by the line arrows. D) Axial MRI image at the T10 level. The injectate enhanced by gadolinium is seen spreading to the intercostal space (line arrows). The neural foramen (arrowhead), paravertebral space (bold arrow), erector spinae muscle (black asterisk), and inferior articular process (white asterisk) are also shown in the diagram. Printed with permission from Philip Peng Educational Series
The sensory levels to pinprick and cold in all six patients are summarized in Fig. 4.
Extent of sensory block in the ventral (F) and dorsal (B) thoracic or abdominal wall. Sensory block at the mid-clavicular line was used to indicate anterior thoraco-abdominal wall spread (V), and sensory block at the interscapular line was used to indicate posterior wall spread (D). D = dorsal; V = ventral
All six patients had complete pain relief (NRS = 0) 30 min after the ESP block. The median [IQR] pre-block NRS at rest and with movement were 8 [6–9] and 10 [9–10], respectively.
Discussion
As opposed to previously reported cadaveric studies,9,10,11,12,13 this was an in vivo MRI study of injectate spread of the ESP block in living patients. We showed that there was consistent spread of injectate into the intercostal space, neural foramina, and the erector spinae muscles, thus contributing to the sensory findings in the ventral and dorsal thoracic and abdominal walls. Nevertheless, the extent of the cephalocaudal spread and the sensory block to the anterior abdominal and thoracic walls were highly variable (Figs 2 and 3).
Since it was first published, ESP block had been one of the most popular regional anesthesia techniques described in the literature.2,17 Multiple randomized-controlled trials have also confirmed the analgesic efficacy of this block.2,3 Despite its popularity, the mechanism of ESP block action is unclear. The popular belief is that the ESP block leads to LA injectate crossing the superior costotransverse ligament and spreading into the paravertebral space.11 This anterior spread is the basis for the blockade of the ventral rami resulting in thoracic (or abdominal) analgesic effects. Five studies, all in cadavers, examined the spread, but the results were conflicting. Spread to the paravertebral space was minimal to none in two studies,12,13 with no spread to the intercostal nerves in three studies.10,12,13 Four studies used anatomical dissection to examine the spread,9,10,12,13 one assessed the spread with three-dimensional computed tomography scan,13 and another one with MRI.11 The volume of injectate was 20 mL in all but one study (where 30 mL of injectate was used).13 Nevertheless, the authors of those studies agreed that correlating spread in cadaver models with spread in live human subjects is limited. The ESP is an interfascial plane block with the target between the tip of the transverse process and erector spinae muscles. In live human subjects, the spread can be potentially enhanced by the contraction of the erector spinae muscles and the negative intrathoracic pressure during inspiration (Fig. 5). These mechanisms potentially enhance spread towards the paravertebral and intercostal spaces.8,14 Thus, examining the injectate spread in live human subjects can further enhance our understanding of the mechanism of ESP block in clinical settings.
Schematic diagram of the possible mechanism potentiating the spread of injectate in erector spinae (ES) plane block. The middle diagram is the sagittal section of the thoracic spine. The left insert shows the negative intrathoracic pressure drawing local anesthetic (LA) towards the paravertebral space. The right insert shows the erector muscles contraction “pushing” the LA in the erector spinae plane towards the paravertebral space. The artwork is produced by Dr. Vicente Roques (imedar.com) and is printed with permission from Dr. Roques
In the present study, consistent sensory block in the posterior (dorsal) thoracic and abdominal wall was evident, and was supported by the extensive spread of injectate in the erector spinae muscles through which the posterior rami transit. Nevertheless, the cephalocaudal extent of the sensory block to the anterior (ventral) thoracic and abdominal walls was much more variable although it was present in all patients. Though it is not feasible to perform a correlation analysis with such a small number of patients, the spread patterns seen with MRI (i.e., with spread to neural foramina and intercostal nerve distributions) were consistent with the sensory block. The pattern of spread showed that the larger the extent of spread to both the intercostal nerve and neural foramina (e.g., comparing patients 1 and 2 with patients 4 and 5), the more extensive were the sensory levels shown. In two patients (patients 1 and 2), injectate spread to the epidural space, but the significance of this epidural spread was uncertain; it may have been a consequence of extensive injectate spread into the paravertebral space and neural foramina. Thus, the mechanism of sensory blockade of the anterior thoracoabdominal wall by the ESP block appears to be related to spread to the neural foramina and intercostal space. Spread to the epidural space is an unlikely mechanism with 30–35 mL injectate.
Compared with previous cadaver studies on injectate spread following ESP block, our model in live human subjects offers a few advantages. First, it simulates a real-life situation, including the theoretical effects of respiration and muscle contraction on the extent of injectate spread in the erector spinae muscle plane. It also avoids biochemical change of hyaluronic acid, a key substance controlling the viscosity of connective tissue and thus injectate spread. Even in fresh embalmed cadavers, a slight drop in the temperature will increase the viscosity of hyaluronic acid in the extracellular matrix and affect connective tissue in the fascial plane. This could be relevant to the spread of injectate to the intercostal nerves,18,19 and may account for the negative spread to the intercostal space observed in three previous cadaver studies (while our study consistently showed extensive spread to the intercostal nerve).
A second difference in our study compared with those published previously is that we added gadolinium to the injectate. Nevertheless, using MRI to track the LA fluid spread following the injection can be challenging.20 The fluid signal is usually hypointense in the T1-weighted sequence. To accentuate the visualization of water content, a T2-weighted sequence is usually used, but it can be difficult to differentiate the fluid content from cerebrospinal fluid, blood vessels, and soft tissue. Gadolinium has an odd number of electrons, which confers it considerable paramagnetic power and renders it hyperintense by increasing the intensity of the magnetic field around it. Thus, by mixing gadolinium with LA, the signal of the mixture is accentuated and can be tracked with just a T1 sequence; this can differentiate it from cerebrospinal fluid in the spinal canal.21
We also correlated MRI findings with sensory evaluations and pain relief. Spreading of the contrast in MRI studies (or dye in cadavers) to any nerves may not be clinically significant because of the concentration effect. On the other hand, when intact nerves are exposed to LA with a high pKa (e.g., ropivacaine and bupivacaine) at lower concentrations, the C fibres are consistently blocked ahead of the myelinated A fibres. Therefore, the spread may not be detected by the usual sensory test, which in turn may underestimate the clinical analgesic effects.22 By correlating with sensory assessment and pain relief in live subjects, the clinician can better appreciate the clinical significance of the spread.
A limitation of this study was the sample size. Even with a single physician performing all blocks at the same level on the same side, the extent of spread and the sensory effects were quite variable. A larger sample size might allow for a better appreciation of the relationship between the sensory effects and injectate spread. Nevertheless, the recruitment of patients was very difficult, especially because an extra MRI procedure was required.
In summary, our novel study examining injectate spread in live patients following the ESP block showed that the LA injectate consistently spread to the dorsal rami among the erector spinae muscle resulting in posterior thoracic and abdominal wall blockade. We also showed that the LA injectate consistently spread to the neural foramina and into the intercostal space, contributing to a clinically meaningful sensory change and pain relief in the anterior (ventral) thoracic and abdominal walls. Nevertheless, the extent of spread to the neural foramina and intercostal space was highly variable. Lastly, although the full clinical impact is not known, the LA injectate spread to the epidural space in some patients, so clinicians need to exercise caution when using high volumes of LA during ESP blockade.
References
Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med 2016; 41: 621-7.
Uppal V, Ip VH. Curb Your Enthusiasm. Erector Spinae Plane Block - Because It Is Easy’ Is Not a Good Reason To Do It. ASRA Newsletter - November 2019. Available from URL: https://www.asra.com/asra-news/article/213/curb-your-enthusiasm-erector-spinae-plan (accessed February 2020).
Forero M, Roqués V, Trujillo-Uribe NJ. Erector spinae plane block. In: Peng P, Finlayson R, Lee SH, Bhatia A (Eds). Ultrasound or Interventional Pain Management. An Illustrated Procedural Guide. Switzerland: Springer; 2019: 131-48.
Abu Elyazed MM, Mostafa SF, Abdelghany MS, Eid GM. Ultrasound-guided erector spinae plane block in patients undergoing open epigastric hernia repair: a prospective randomized controlled study. Anesth Analg 2019; 129: 235-40.
Nagaraja PS, Ragavendran S, Singh NG, et al. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth 2018; 21: 323-7.
Forero M, Rajarathinam M, Adhikary SD, Chin KJ. Erector spinae plane block for the management of chronic shoulder pain: a case report. Can J Anesth 2018; 65: 288-93.
Tulgar S, Kose HC, Selvi O, et al. Comparison of ultrasound-guided lumbar erector spinae plane block and transmucular quadratus lumborum block for postoperative analgesia in hip and proximal femur surgery: a prospective randomized feasibility study. Anesth Essays Res 2018; 12: 825-31.
Sondekoppam RV, Tsui BC. “Minimally invasive” regional anesthesia and the expanding use of interfascial plane blocks: the need for more systematic evaluation. Can J Anesth 2019; 66: 855-63.
Vidal E, Gimenez H, Forero M, Fajardo M. Erector spinae plane block: a cadaver study to determine its mechanism of action. Rev Esp Anestesiol Reanim 2018; 65: 514-9.
Yang HM, Choi yJ, Kwon HJ, Cho TH, Kim SH. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia 2018; 73: 1244-50.
Adhikary SD, Bernard S, Lopez H, Chin KJ. Erector spinae plane block versus retrolaminar block: a magnetic resonance imaging and anatomical study. Reg Anesth Pain Med 2018; 43: 756-62.
Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med 2018; 43: 567-71.
Aponte A, Sala-Blanch X, Prats-Galino A, Masdeu J, Moreno LA, Sermeus LA. Anatomical evaluation of the extent of spread in the erector spinae plane block: a cadaveric study. Can J Anesth 2019; 66: 886-93.
Behr AU, Chan VW, Stecco C. Living versus cadaver fascial plane injection. Reg Anesth Pain Med 2019; DOI: https://doi.org/10.1136/rapm-2019-100893.
Schwartzmann A, Peng P, Maciel MA, Forero M. Mechanism of the erector spinae plane block: insights from a magnetic resonance imaging study. Can J Anesth 2018; 65: 1165-6.
Chin KJ, Karmakar MK, Peng P. Ultrasonography of the adult thoracic and lumbar spine for central neuraxial blockade. Anesthesiology 2011; 114: 1459-85.
Tsui BC, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: a pooled review of 242 cases. J Clin Anesth 2019; 53: 29-34.
Stecco C. Functional Atlas of the Human Fascial System. Elsevier, 2015.
Pavan PG, Stecco A, Stern R, Stecco C. Painful connections: densification versus fibrosis of fascia. Curr Pain Headache Rep 2014; DOI: https://doi.org/10.1007/s11916-014-0441-4.
Vermeylen K, Desmet M, Leunen I. Supra-inguinal injection for fascia iliaca compartment block results in more consistent spread towards the lumbar plexus than an infra-inguinal injection: a volunteer study. Reg Anesth Pain Med 2019; DOI: https://doi.org/10.1136/rapm-2018-100092.
Carrasco Munoz S, Calles Blanco C, Marcin J, Fernández AC, Lafuente Martinez J. Gadolinium-based contrast agents for magnetic resonance imaging. Radiology 2014; 56 (Suppl 1): 21-8.
Ford DJ, Raj PP, Singh P, Regan KM, Ohlweiler D. Differential peripheral nerve block by local anesthetics in the cat. Anesthesiology 1984; 60: 28-33.
Author contributions
Philip Peng, Mariano Antunez Maciel, Ana Schwartzmann, Mauricio Forero, Paola Alcarraz, and **mena Gonzalez contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article.
Conflicts of interest
None.
Funding statement
None.
Disclosure
Philip Peng: Equipment support from Sonosite Fujifilm Canada. The study, including the cost of magnetic resonance imaging, was supported by institutional funding from Hospital de Clinicas, Universidad de la Republica, Montevideo, Uruguay.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schwartzmann, A., Peng, P., Maciel, M.A. et al. A magnetic resonance imaging study of local anesthetic spread in patients receiving an erector spinae plane block. Can J Anesth/J Can Anesth 67, 942–948 (2020). https://doi.org/10.1007/s12630-020-01613-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-020-01613-8