Abstract
Background
Malignant hyperthermia (MH) is a potentially lethal disorder of skeletal muscle triggered by anesthetic agents. A histomorphological examination of diseased muscle may provide insight into MH pathophysiology, but it is not a routine part of standard-of-care practice for the identification of MH-susceptibility. In this study, we investigated muscle histomorphology in a large cohort of MH-susceptible (MHS) patients and examined its relationship to genotype and phenotype.
Methods
All consenting patients who were identified as MHS based on a caffeine-halothane contracture test (CHCT) performed during 1992-2011 were retrospectively identified and recruited for this study. Results of the histomorphological examination, which is a routine part of our centre-specific practice, were reviewed. Patient demographics, MH proband status, histological features, CHCTs, and genetic results for MH-causative mutations were summarized.
Results
Seven of the 399 patients classified as MHS had histological characteristics consistent with central core disease, and one patient was a carrier of Duchenne’s muscular dystrophy. Eighty-six (22%) patients had histological abnormalities, and five (6%) of these had evidence of “frank” myopathy. No histologic abnormalities were consistent among the MHS patients; however, a higher proportion of MH probands had abnormal histomorphology compared with the general MHS population, and patients with evidence of “frank” myopathy showed similarities in clinical history, biochemistry, CHCT, and genetic testing.
Conclusion
Despite the inability of the histomorphological examination to identify consistent features in MHS patients, histology may serve as a potential adjunct to CHCT and aid in the identification of other myopathies. Nevertheless, the specifics of its utility ought to be assessed in other studies and by way of formal cost-effectiveness analysis.
Résumé
Contexte
L’hyperthermie maligne (HM) est un trouble potentiellement fatal des muscles squelettiques déclenché par les agents anesthésiques. Un examen histomorphologique du muscle atteint pourrait nous donner un aperçu de la physiopathologie de l’HM, mais il ne fait pas partie de la pratique normale de soins pour l’identification d’une susceptibilité à l’HM. Dans cette étude, nous avons examiné l’histomorphologie musculaire chez une grande cohorte de patients susceptibles à l’HM (SHM) et sa relation au génotype et au phénotype.
Méthode
Tous les patients consentants identifiés comme SHM selon un test de contracture à la caféine et à l’halothane (CHCT) réalisé entre 1992 et 2011 ont été identifiés rétrospectivement puis recrutés pour cette étude. Les résultats de l’examen histomorphologique, une partie intégrante de la pratique spécifique de notre centre, ont été passés en revue. Les données démographiques des patients, leur statut de proposant de HM, les traits histologiques, les CHCT et les résultats génétiques de mutations causatives de HM ont été résumés.
Résultats
Parmi les 399 patients classés comme SHM, sept patients possédaient des caractéristiques histologiques correspondant à une myopathie congénitale à axe central, et un patient était porteur d’une dystrophie musculaire de Duchenne. Quatre-vingt-six (22 %) patients présentaient des anomalies histologiques, et cinq (6 %) de ces patients montraient des signes de myopathie « franche ». Aucune anomalie histologique n’a été trouvée de façon constante chez les patients SHM; toutefois, une proportion plus élevée de proposants d’HM présentait une histomorphologie anormale comparativement à la population SHM générale, et les patients montrant des signes de myopathie « franche » présentaient des similitudes en matière d’antécédents cliniques, de biochimie, de CHCT et de test génétique.
Conclusion
Malgré l’incapacité de l’examen histomorphologique à identifier des caractéristiques constantes chez les patients SHM, l’histologie pourrait servir d’ajout potentiel au test de CHCT et aider à identifier d’autres myopathies. Toutefois, les détails de son utilité devraient être évalués dans d’autres études et dans le cadre d’une analyse formelle de coût-efficacité.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Malignant hyperthermia (MH) is an inherited disorder caused by dysregulation of calcium homeostasis in skeletal muscle cells.1 In susceptible individuals, it is usually triggered by commonly used volatile anesthetic agents and succinylcholine and can result in severe hyperpyrexia, acid-base disturbances, and perioperative death.2,3 Although timely preoperative identification of these individuals is warranted to avert such adverse outcomes, the heterogeneous pheno- and genotypic nature of the disorder presents inherent challenges. One area of interest in the effort to understand MH pathophysiology and improve perioperative identification and treatment involves exploration of the interrelationships between functional and structural characteristics of diseased muscle.4–6
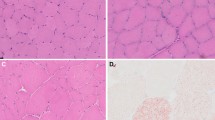
a) An H&E-stained photomicrograph from a muscle biopsy of a 29-yr-old female identified as MHS. The histology is normal with no evidence of fibrosis, variability in myofibre size, necrosis, inclusions, or myonuclear internalization. b) A myosin F immunohistochemistry specimen of a 51-yr-old male identified as MHS, showing evidence of type II fibre atrophy. Notice the relatively smaller size of type II fibres (II; dark grey) compared with type I fibres (I; light grey). c) An H&E-stained snap-frozen muscle sample of a 39-yr old male identified as MHS, showing significantly increased variability in fibre size and increased internalization of myonuclei – two components of “frank” myopathic change (necrotic and regenerating fibres and interstitial fibrosis are not shown)
The standard method for assessing MH susceptibility in North America is the caffeine-halothane contracture test (CHCT), and the in vitro contracture test (IVCT) is used elsewhere; both tests are performed on a muscle biopsy. The CHCT allows for classification of patients as MH-susceptible (MHS) and MH-normal (MHN) on the basis of contracture response which reflects muscle function. In various studies, patterns of structural histomorphological abnormalities and their relationship to the functional classification have been examined. Whereas a few studies have reported a significantly increased proportion of distinct histomorphological characteristics in MHS patients,4,7 others have failed to find such relationships.8–11 In the current retrospective study, we sought to investigate the utility and value of a routine histologic examination for MH diagnosis in a large at-risk population of patients referred for a CHCT. Our secondary objective was to investigate histomorphological characteristics in MHS patients and the associations between phenotype (both functional and structural) and genotype.
Methods
Patient population and the CHCT
Following institutional Research Ethics Board (REB) approval, all consenting patients who underwent a CHCT at the Malignant Hyperthermia Investigation Unit at Toronto General Hospital (Toronto, Canada) during 1992-2011 were retrospectively identified and recruited for the study. The CHCTs were performed in accordance with the standardized North American MH protocol,12 thus facilitating comparison of results across the study period. A contracture of ≥ 0.7 g to 3% halothane or a contracture of ≥ 0.3 g to 2.0 mM caffeine in at least one muscle fascicle conferred MHS status.
Data abstraction included patient age, sex, MH proband status (defined as any patient who was documented to have an adverse anesthetic reaction at the time of referral), nonspecific muscle symptoms at the time of referral (defined as muscle pains or cramps/spasms and/or weakness), and the results of the CHCT, histomorphological examination, and genetic testing. When applicable, the severity of each adverse anesthetic reaction was evaluated using the clinical grading scale (CGS).13 “Very likely” MH events were defined by scores of 35-49 and “almost certain” events were defined by scores of ≥ 50. Patients with central core disease (CCD) were referred primarily due to muscle symptoms and were not included in the analyses due to known adverse reactions under anesthesia and their representation of a disease process distinct from MH. The variations in phenotype (MH proband status, CHCT results, nonspecific muscle symptoms) and genotype (genetic testing for MH-causative mutations) in relation to muscle histomorphology were the primary relationships of interest.
Histomorphological examination
A histological examination is routinely performed for all MHS patients as part of our centre-specific practice. A single neuropathologist (J.K.) who was aware of patients’ MHS status but blinded to the original histological impression, patient demographics, MH proband status, and genetic results conducted a central pathology review by retrieving and re-examining the slides from the original muscle biopsies of all MHS patients whose original pathology report described some morphologic abnormality. For each specimen, the reviewing neuropathologist noted the presence of the following abnormalities on microscopic examination: 1) presence of increased variability in myofibre size; 2) type II fibre atrophy; 3) increased myonuclear internalization (defined as greater than in approximately 5% of fibres); 4) necrotic fibres; 5) regenerating fibres; 6) interstitial fibrosis; 7) central cores; and 8) any other obvious abnormalities – all of which have been previously described in MHS patients.4,7,11 Further subclassification was made into “mild” myopathic changes (defined by the presence and extent of fibre size variability and myonuclear internalization) and “frank” myopathic changes (defined by the presence and extent of fibre size variability, fibrosis, myonuclear internalization, and necrotic/regenerating fibres). For all samples, conventional histologic stains included hematoxylin and eosin (H&E), Gomori trichrome, nicotinamide adenine dinucleotide + hydrogen (NADH), and at least one pH-specific ATPase. Some of the original specimens had also been treated with additional enzymology or special stains, including Congo red, Oil Red O, periodic acid-Schiff (PAS), phosphorylase, and/or acid phosphatase. In cases where the slides from the original biopsy were unavailable for central pathology review, information from the original pathology reports was utilized for analysis.
Genetic testing
Following separate REB approval, genetic testing for 30 known causative ryanodine receptor 1 (RYR1) mutations14 was performed for all consenting MHS patients. Genomic DNA was extracted from patients’ blood leukocytes according to published procedures.15 RYR1 exons were amplified via the polymerase chain reaction (PCR) as described previously.16 Sequencing reactions were performed at the DNA Sequencing and Synthesis Facility of The Centre for Applied Genomics (Toronto, ON, Canada). Raw sequence data analysis (contig building and sequence comparison with the reference RYR1 sequence, GenBank accession NC 000019) was carried out using Sequencher 4.10.1 (Gene Codes, Ann Arbor, MA, USA).
Statistical analysis
Descriptive statistics were used to summarize patient characteristics, CHCT results, and the results of RYR1 mutation testing for patients with normal and abnormal muscle histomorphology. Continuous data were reported as mean (SD) while categorical data were reported as frequencies or percentages. Independent Student’s t tests and Chi square (or Fisher’s exact) tests were used to compare continuous and categorical variables, respectively, for patients with and without histological abnormalities. All analyses were performed with SAS™ version 9.3 (SAS Institute, Cary, NC, USA), and a P value < 0.05 was considered statistically significant.
Results
Patient characteristics
Over the course of the study period, 1,256 patients were referred for CHCT; 399 of these were classified as MHS and 857 were classified as MHN. Seven (2%) patients with clinical features and histologic characteristics consistent with CCD were excluded from further analysis; the remaining MHS patients (n = 392) comprised the MHS data set.
Eighty-six (22%) MHS patients had a histologic abnormality described in their original pathology report. In 83 (97%) of these cases, blinded central pathology review corroborated these abnormal findings, while the remaining three specimens were reported to be histologically normal. There were no significant differences in age or sex as a function of abnormalities in histology; however, a higher proportion of MH probands (72% vs 46%) had abnormal histomorphology compared with the whole MHS patient set, χ2 (1, n = 392) = 18.6, P < 0.001 (Table 1).
CHCT results
Most patients diagnosed as MHS (63%) tested positive to both caffeine and halothane on the CHCTs. The remaining patients tested positive to either halothane only (34%) or caffeine only (3%). The proportion of patients classified into each of the above three categories on CHCTs did not differ as a function of abnormalities in histology, χ2 (2, n = 392) = 0.76, NS (Table 1).
Phenotype
Nonspecific muscle symptoms were observed in a minority of MHS patients with and without histomorphological abnormalities. The frequency of these symptoms did not differ as a function of abnormal muscle histology, χ2 (2, n = 392) = 0.73, NS (Table 1).
Genetic testing
Due to challenges with retrospective data collection and patient follow-up, only 226 of the 392 (58%) MHS patients underwent genetic testing. Fifty-two (23%) of the genetically tested patients were MH probands. In 86 (38%) of the genetically tested patients, a causative RYR1 mutation for MH was identified, and 40 (47%) of these were MH probands. Furthermore, 66 (77%) of the patients with causative mutations reacted to both caffeine and halothane, 15 (17%) reacted to caffeine only, and five (6%) reacted to halothane only with CHCTs.
Forty-three (31%) of the 140 (62%) genetically tested MHS patients without evidence of a causative RYR1 mutation carried a variant of unknown significance, and 72 (51%) carried at least one polymorphism in RYR1.
Overall, there was no difference in the prevalence of RYR1 causative mutations as a function of abnormalities in histology, χ2 (1, n = 226) = 2.57, NS (Table 1). Nevertheless, 28 (34%) of the 83 patients with histologic abnormalities were found to have causative RYR1 mutations. The types and frequencies of these causative mutations are described in Table 2.
Abnormalities in histomorphology
Most of the MHS microscopic specimens showed normal histology (Figure a). The most common histologic abnormality was type II fibre atrophy, which was present in more than half of the patients with described histologic aberrations (Figure b). Variability in fibre size, denervation, “mild” myopathic changes, and “frank” myopathic changes were noticed progressively less frequently in the population and constituted the remainder of the five most common abnormalities (Table 1). Necrotic fibres were found in only five samples, and four of these had additional abnormalities (two with “frank” myopathy, one with denervation atrophy, and one with type II fibre atrophy). Three cases had small foci of chronic inflammation as well as increased variability in fibre size; one case had ragged red fibres and one case had tubular aggregates. Excluding the CCD patients, central cores were not seen on light microscopy in any of the analyzed MHS specimens.
“Frank” myopathic changes (Figure c) were noticed in five (6%) patients with abnormalities, with each of these exhibiting all four of the most frequent components of “frank” myopathy (i.e., variability in fibre size, increased myonuclear internalization, necrotic/regenerating fibres, and interstitial fibrosis) in the data set. All of these patients were MH probands with a mean (SD) CGS score of 32 (14) whose anesthetic reactions occurred, on average, more than six months prior to their muscle biopsy. Moreover, all of these patients had CHCT results positive to both caffeine and halothane and confirmed elevations in creatine kinase (CK) as part of the initial CHCT referral, and they presented with nonspecific muscle symptoms at the time of the CHCT. Four (80%) of these patients tested positive for a known causative RYR1 mutation, and one patient was subsequently identified as a carrier of Duchenne’s muscular dystrophy (DMD), which was confirmed with immunohistochemistry (Table 3).
Discussion
Our results show that the histological examinations of 86 MHS patients referred for a CHCT showed strong inter-observer consistency and identified multiple histological abnormalities. The percentage of MHS patients with abnormal histomorphology9,11 and MH causative mutations17–19 was within the range of previous data sets. There were no consistent histological features among MHS patients; however, more MH probands were found in the group with abnormal histomorphology, and a small subset of patients with evidence of “frank” myopathic abnormalities showed multiple similarities involving both historical (clinical history, signs and symptoms, and MH proband status) and biochemical (CK levels) features, CHCT results, and, in the majority of cases, evidence of a known MH-causative mutation in the RYR1 gene.
Type II fibre atrophy was the most common histomorphological abnormality in our data set. This finding is nonspecific and is commonly associated with either disuse atrophy20 or corticosteroid therapy21 but has been described in the context of MH.7,22 Other histologic characteristics, including myonuclear internalization4 and necrosis,7 which have been purported to be due to focal “mini crises” following a MH reaction,23 were uncommon in our patient pool. In all, these results emphasize the histologic variability and lack of consistent features in MHS muscle biopsies.
Genetic testing and CHCT results did not differ between patients with normal findings and those with abnormal histomorphological findings. This observation is not unexpected given the intricacies of gene expression and the complex interrelationships between excitation-contraction coupling (function) and histomorphological features (structure) in MHS skeletal muscle. Incomplete penetrance,24 variable expressivity,4,24 the potential for gene silencing,25 and multiple loci conferring MH susceptibility26 have all been described in the pathophysiology of MH, leading to vast heterogeneity in phenotypic expression. We acknowledge that obstacles with retrospective data collection were a limitation that may have hindered more reliable assessment of the influence of genotype on phenotype in this study. Specifically, the inability to genetically test almost half (42%) of our patient population precluded accurate analysis of this relationship.
Our most interesting and novel observation was the similarity in historical features, biochemical results, and MH-causative mutation testing among the five patients with “frank” myopathic changes in histology. This finding emphasizes the potential utility of the microscopic examination in the identification of a small subset of patients with similar phenotypic and genotypic characteristics of MH in the absence of recent anesthetic triggers. Nevertheless, this assertion is based on the assumption that baseline histologic features remain unchanged following an MH reaction. Although there has been general support for this notion,27,28 the evidence is not entirely consistent.23 Given that all five of our patients with “frank” myopathic changes were MH probands, it is impossible to classify histologic abnormalities as either primary (inherent characteristic of the muscle) or secondary (new changes following the MH reaction) based on our data set. Similarly, the cause of the increased prevalence of abnormal histomorphology among MH probands is less discernible in the absence of a baseline histologic examination prior to the anesthetic reaction. Nevertheless, these findings suggest that the histomorphological examination may serve as a potential adjunct to CHCT for MHS patients.
At present, inclusion of a histologic examination reflects centre-dependent practice for the identification of MH susceptibility.29 Nevertheless, it may aid in the identification of other inherited myopathies, thus facilitating their diagnosis and management. In the current study, one patient was diagnosed with DMD (which may have implications for family planning and unique anesthetic considerations), and seven others were found to have CCD. Other studies have reported the diagnosis of six and seven cases of specific myopathies following the histologic evaluation of 132 and 33 MHS patients, respectively.9,11 There were no cases of immune-mediated (and thus potentially treatable)30 myositides identified in our MHS patient cohort. In all, the potential benefits of the histologic examination need to be justified against the added costs incurred.
In summary, in this single-centre study, we examined the relationships between histologic features, caffeine-halothane contracture tests, MH proband status, and genotype in a large population of patients referred for CHCTs. Results suggest that despite the inability of the microscopic examination to identify consistent histologic features in MHS patients, histology may serve as a potential adjunct to CHCT and aid in the identification of other myopathies. Even so, the specifics of its use ought to be assessed in other large studies and by way of formal cost-effectiveness analysis. Only then could accurate evidence-based recommendations on its utility in MHS patients be formally made.
References
Denborough MA, Lovell RR. Anaesthetic deaths in a family. Lancet 1960; 2: 45.
Rosero EB, Adesanya AO, Timaran CH, Joshi GP. Trends and outcomes of malignant hyperthermia in the United States, 2000 to 2005. Anesthesiology 2009; 110: 89-94.
Rosenbaum HK, Miller JD. Malignant hyperthermia and myotonic disorders. Anesthesiol Clin North America 2002; 20: 623-64.
Harriman DG. Malignant hyperthermia myopathy–a critical review. Br J Anaesth 1988; 60: 309-16.
Reske-Nielsen E, Haase J, Kelstrup J. Malignant hyperthermia in a family. The neurophysiological and light microscopical study of muscle biopsies of healthy members. Acta Pathol Microbiol Scand A 1975; 83: 645-50.
Gullotta F, Spiess-Kiefer C. Muscle biopsy studies in malignant hyperthermia (German). Anasth Intensivther Notfallmed 1983; 18: 21-7.
Mezin P, Payen JF, Bosson JL, Brambilla E, Stieglitz P. Histological support for the difference between malignant hyperthermia susceptible (MHS), equivocal (MHE) and negative (MHN) muscle biopsies. Br J Anaesth 1997; 79: 327-31.
Ranklev E, Henriksson KG, Fletcher R, Germundsson K, Oldfors A, Kalimo H. Clinical and muscle biopsy findings in malignant hyperthermia susceptibility. Acta Neurol Scand 1986; 74: 452-9.
Figarella-Branger D, Kozak-Ribbens G, Rodet L, et al. Pathological findings in 165 patients explored for malignant hyperthermia susceptibility. Neuromuscul Disord 1993; 3: 553-6.
Heiman-Patterson T, Fletcher JE, Rosenberg H, Tahmoush AJ. No relationship between fiber type and halothane contracture test results in malignant hyperthermia. Anesthesiology 1987; 67: 82-4.
von Breunig F, Wappler F, Hagel C, et al. Histomorphologic examination of skeletal muscle preparations does not differentiate between malignant hyperthermia-susceptible and -normal patients. Anesthesiology 2004; 100: 789-94.
Larach MG. Standardization of the caffeine halothane muscle contracture test. North American Malignant Hyperthermia Group. Anesth Analg 1989; 69: 511-5.
Larach MG, Localio AR, Allen GC, et al. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology 1994; 80: 771-9.
European Malignant Hyperthermia Group. Causative RYR1 mutations. Basel, Switzerland. Available from URL: http://www.emhg.org/genetics/mutations-in-ryr1/ (accessed June 2013).
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215.
Kraev N, Loke JC, Kraev A, MacLennan DH. Protocol for the sequence analysis of ryanodine receptor subtype 1 gene transcripts from human leukocytes. Anesthesiology 2003; 99: 289-96.
Kraeva N, Riazi S, Loke J, et al. Ryanodine receptor type 1 gene mutations found in the Canadian malignant hyperthermia population. Can J Anesth 2011; 58: 504-13.
Brandom BW, Bina S, Wong CA, et al. Ryanodine receptor type 1 gene variants in the malignant hyperthermia-susceptible population of the United States. Anesth Analg 2013; 116: 1078-86.
Sambuughin N, Holley H, Muldoon S, et al. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the North American population. Anesthesiology 2005; 102: 515-21.
Engel WK. Selective and nonselective susceptibility of muscle fiber types. A new approach to human neuromuscular diseases. Arch Neurol 1970; 22: 97-117.
Kanda F, Okuda S, Matsushita T, Takatani K, Kimura KI, Chihara K. Steroid myopathy: pathogenesis and effects of growth hormone and insulin-like growth factor-I administration. Horm Res 2001; 56(Suppl 1): 24-8.
Ellis FR. Malignant hyperthermia. In: Ellis FR (Ed.). Inherited Disease and Anaesthesia Elsevier/North-Holland Biomedical Press; 1981: 163-211.
Carpenter S, Karpati G. Pathology of skeletal muscle, 2nd ed. Oxford University Press; 2001.
Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat 2006; 27: 977-89.
Lu Q, Qiu X, Hu N, Wen H, Su Y, Richardson BC. Epigenetics, disease, and therapeutic interventions. Ageing Res Rev 2006; 5: 449-67.
Robinson R, Hopkins P, Carsana A, et al. Several interacting genes influence the malignant hyperthermia phenotype. Hum Genet 2003; 112: 217-8.
Harriman DG, Sumner DW, Ellis FR. Malignant hyperpyrexia myopathy. Q J Med 1973; 42: 639-64.
Harriman DG. Preanesthetic investigation of malignant hyperthermia: microscopy. Int Anesthesiol Clin 1979; 17: 97-117.
Rosenberg H, Antognini JF, Muldoon S. Testing for malignant hyperthermia. Anesthesiology 2002; 96: 232-7.
Miller FW. New approaches to the assessment and treatment of the idiopathic inflammatory myopathies. Ann Rheum Dis 2012; 71(Suppl 2): i82-5.
Conflicts of interest
None declared.
Funding
This work was funded by the Department of Anesthesia, University of Toronto Merit Award to Sheila Riazi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
David Orlov and Sheila Riazi participated in the analysis and interpretation of data. David Orlov wrote the original draft. David Orlov, Julia Keith, Derek Rosen, Sidney Croul, and Sheila Riazi were involved with the critical revision of the original manuscript. Julia Keith, Derek Rosen, Sidney Croul, Natalia Kraeva, and Sheila Riazi were involved with the acquisition of data. Julia Keith and Sidney Croul were involved with the analysis and interpretation of the histomorphological results. Derek Rosen, Natalia Kraeva, and Sheila Riazi were involved with the conception and design of the study.
Rights and permissions
About this article
Cite this article
Orlov, D., Keith, J., Rosen, D. et al. Analysis of histomorphology in malignant hyperthermia-susceptible patients. Can J Anesth/J Can Anesth 60, 982–989 (2013). https://doi.org/10.1007/s12630-013-0005-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-0005-9