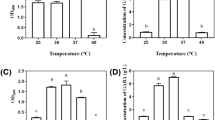
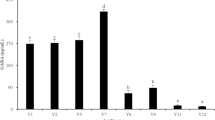
Abstract
Gamma-aminobutyric acid (GABA) is a principal inhibitory neurotransmitter in the central nervous system and is produced by irreversible decarboxylation of glutamate. It possesses several physiological functions such as neurotransmission, diuretic, and tranquilizer effects and also regulates cardiovascular functions such as blood pressure and heart rate in addition to playing a role in the reduction of pain and anxiety. The objective of this study was to evaluate the GABA producing ability and probiotic capability of certain lactic acid bacteria strains isolated from dairy products. Around sixty-four bacterial isolates were collected and screened for their ability to produce GABA from monosodium glutamate, among which nine isolates were able to produce GABA. The most efficient GABA producer was Enterococcus faecium BS5. Further, assessment of several important and desirable probiotic properties showed that Ent. faecium BS5 was resistant to acid stress, bile salt, and antibiotics. Ent. faecium BS5 may potentially be used for large-scale industrial production of GABA and also for functional fermented product development.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Huang J, Mei L, Sheng Q, Yao S, Lin D (2007) Purification and characterization of glutamate decarboxylase of Lactobacillus brevis CGMCC 1306 isolated from fresh milk. Chin J Chem Eng 15(2):157–161. https://doi.org/10.1016/S1004-9541(07)60051-2
Hayakawa K, Kimura M, Yamori Y (2005) Role of the renal nerves in γ-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur J Pharmacol 524:120–125. https://doi.org/10.1016/j.ejphar.2005.09.020
Ueno H (2000) Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B Enzym 10:67–79. https://doi.org/10.1016/S1381-1177(00)00114-4
Vaiva G, Thomas P, Ducrocq F et al (2004) Low posttrauma GABA plasma levels as a predictive factor in the development of acute posttraumatic stress disorder. Biol Psychiatry 55:250–254. https://doi.org/10.1016/j.biopsych.2003.08.009
Park KB, Oh SH (2007) Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour Technol 98:1675–1679. https://doi.org/10.1016/j.biortech.2006.06.006
Soltani N, Qiu H, Aleksic M et al (2011) GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci U S A 108(28):11692–11697. https://doi.org/10.1073/pnas.1102715108
Pokusaeva K, Johnson C, Luk B et al (2016) GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil 29: 1–14. https://doi.org/10.1111/nmo.12904
Auteri M, Zizzo MG, Serio R (2015) GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res 93:11–21. https://doi.org/10.1016/j.phrs.2014.12.001
Chen S, Tan B, **a Y et al (2018) Effects of dietary gamma-aminobutyric acid supplementation on the intestinal functions in weaning piglets. Food Funct 10:366–378. https://doi.org/10.1039/c8fo02161a
Zhao Y, Wang J, Wang H et al (2020) Effects of GABA supplementation on intestinal SIgA secretion and gut microbiota in the healthy and ETEC-infected weanling piglets. Mediators Inflamm 2020:1–17. https://doi.org/10.1155/2020/7368483
Kim JY, Lee MY, Ji GE, Lee YS, Hwang KT (2009) Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int J Food Microbiol 130:12–16. https://doi.org/10.1016/j.ijfoodmicro.2008.12.028
Thwe SM, Kobayashi T, Luan T, Shirai T, Onodera M, Hamada-Sato N, Imada C (2011) Isolation, characterization, and utilization of γ-aminobutyric acid (GABA)-producing lactic acid bacteria from Myanmar fishery products fermented with boiled rice. Fish Sci 77:279–288. https://doi.org/10.1007/s12562-011-0328-9
Seo MJ, Lee JY, Nam YD et al (2013) Production of γ-aminobutyric acid by Lactobacillus brevis 340G isolated from kimchi and its application to skim milk. Food Eng Prog 17(4):418–423. https://doi.org/10.13050/foodengprog.2013.17.4.418
Binh TTT, Ju WT, Jung WJ, Park RD (2014) Optimization of γ-aminobutyric acid production in a newly isolated Lactobacillus brevis. Biotechnol Lett 36:93–98. https://doi.org/10.1007/s10529-013-1326-z
Lim HS, Cha IT, Roh SW, Shin HH, Seo MJ (2017) Enhanced production of gamma-aminobutyric acid by optimizing culture conditions of Lactobacillus brevis HYE1 isolated from kimchi, a Korean fermented food. J Microbiol Biotechnol 27(3):450–459. https://doi.org/10.4014/jmb.1610.10008
Huang J, Mei LH, Wu H, Lin DQ (2007) Biosynthesis of γ-aminobutyric acid (GABA) using immobilized whole cells of Lactobacillus brevis. World J Microbiol Biotechnol 23:865–871. https://doi.org/10.1007/s11274-006-9311-5
Komatsuzaki N, Shima J, Kawamoto S, Momose H, Kimura T (2005) Production of γ-aminobutyric acid by Lactobacillus paracaesai isolated from traditional fermented foods. Food Microbiol 22:497–504. https://doi.org/10.1016/j.fm.2005.01.002
Park SY, Lee JW, Lim SD (2014) The probiotic characteristics and GABA production of Lactobacillus plantarum K154 isolated from kimchi. Food Sci Biotechnol 23(6):1951–1957. https://doi.org/10.1007/s10068-014-0266-2
Sa HD, Park JY, Jeong SJ, Lee KW, Kim JH (2015) Characterization of glutamate decarboxylase (GAD) from Lactobacillus sakei A156 isolated from jeot-gal. J Microbiol Biotechnol 25(5):696–703. https://doi.org/10.4014/jmb.1412.12075
Lu XX, **e C, Gu ZX (2009) Optimisation of fermentative parameters for GABA enrichment by Lactococcus lactis. Czech J Food Sci 27(6):433–442
Gran HM, Gadaga HT, Narvhus JA (2003) Utilization of various starter cultures in the production of amasi, a Zimbabwean naturally fermented raw milk product. Int J Food Microbiol 88:19–28. https://doi.org/10.1016/s0168-1605(03)00078-3
Mancini A, Carafa I, Franciosi E, Nardin T, Bottari B, Larcher R, Tuohy KM (2019) In vitro probiotic characterization of high GABA producing strain Lactobacillus brevis DSM 32386 isolated from traditional “wild” alpine cheese. Ann Microbiol 69:1435–1443. https://doi.org/10.1007/s13213-019-01527-x
Sanchart C, Rattanaporn O, Haltrich D, Phukpattaranont P, Maneerat S (2016) Technological and safety properties of newly isolated GABA-producing Lactobacillus futsaii strains. J Appl Microbiol 121(3):734–745. https://doi.org/10.1111/jam.13168
Foulquié Moreno MR, Sarantinopoulos P, Tsakalidou E, DeVuyst L (2006) The role and application of enterococci in food and health. Int J Food Microbiol 106:1–24. https://doi.org/10.1016/j.ijfoodmicro.2005.06.026
Kimoto-Nira H, Suzuki S, Suganuma H, Moriya N, Suzuki C (2015) Growth characteristics of Lactobacillus brevis KB290 in the presence of bile. Anaerobe 35:96–101. https://doi.org/10.1016/j.anaerobe.2015.08.001
Maassen CBM, Claassen E (2008) Strain-dependent effects of probiotic lactobacilli on EAE autoimmunity. Vaccine 26:2056–2057. https://doi.org/10.1016/j.vaccine.2008.02.035
Cani PD, Van Hul M (2015) Novel opportunities for next-generation probiotics targeting metabolic syndrome. Curr Opin Biotech 32:21–27. https://doi.org/10.1016/j.copbio.2014.10.006
Das D, Goyal A (2015) Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT - Food Sci Technol 61:263–268. https://doi.org/10.1016/j.lwt.2014.11.013
Choi SI, Lee JW, Park SM, Lee MY, JI GE, Park MS, Heo TR, (2006) Improvement of gamma-aminobutyric acid (GABA) production using cell entrapment of Lactobacillus brevis GABA 057. J Microbiol Biotechnol 16(4):562–568
Kim MJ, Kim KS (2012) Isolation and identification of γ-aminobutyric acid (GABA) producing lactic acid bacteria from kimchi. J Korean Soc Appl Biol Chem 55:777–785. https://doi.org/10.1007/s13765-012-2174-6
Yang SY, Lü FX, Lu ZX, Bie XM, Jiao Y, Sun LJ, Yu B (2008) Production of γ-aminobutyric acid by Streptococcus salivarius subsp. thermophilus Y2 under submerged fermentation. Amino Acids 34:473–478. https://doi.org/10.1007/s00726-007-0544-x
Pradeep SR, Malleshi NG, Guha M (2011) Germinated millets and legumes as a source of gamma-aminobutyric acid. World appl Sci 14:108–113
Rossetti V, Lombard A (1996) Determination of glutamate decarboxylase by high-performance liquid chromatography. J Chromatogr B 681: 63–67. https://doi.org/10.1016/0378-4347(96)88202-8
Dussault HP (1955) An improved technique for staining red halophilic bacteria. J Bacteriol 70(4):484–485
Sambrook J, Russel DW (2001) Molecular cloning: A laboratory manual. Cold spring harbor laboratory press, New York
Kumar S, Tamura K, Nei M (2004) MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5(2):150–163. https://doi.org/10.1093/bib/5.2.150
Ahire JJ, Patil KP, Chaudhari BL, Chincholkar SB (2011) A potential probiotic culture ST2 produces siderophore 2,3-dihydroxybenzoylserine under intestinal conditions. Food Chem 127:387–393. https://doi.org/10.1016/j.foodchem.2010.12.126
Thapa N, Pal J, Tamang JP (2004) Microbial diversity in ngari, hentak, and tungtap, fermented fish products of north-east India. World J Microbiol Biotechnol 20:599–607. https://doi.org/10.1023/B:WIBI.0000043171.91027.7e
Patel AK, Ahire JJ, Pawar SP, Chaudhari BL, Chincholkar SB (2009) Comparative accounts of probiotic characteristics of Bacillus spp. isolated from food wastes. Food Res Int 42:505–510. https://doi.org/10.1016/j.foodres.2009.01.013
Abedi J, Saatloo MV, Nejati V, Hobbenaghi R et al (2018) Selenium-enriched Saccharomyces cerevisiae reduces the progression of colorectal cancer. Biol Trace Elem Res 185:424–432. https://doi.org/10.1007/s12011-018-1270-9
Thankappan B, Ramesh D, Ramkumar S, Natarajaseenivasan K, Anbarasu K (2015) Characterization of Bacillus spp. from the gastrointestinal tract of labeo rohita—towards to identify novel probiotics against fish pathogens. Appl Biochem Biotechnol 175:340–353. https://doi.org/10.1007/s12010-014-1270-y
Cheikhyoussef A, Pogori N, Chen H et al (2009) Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances (BLIS) produced by Bifidobacterium infantis BCRC 14602. Food Control 20:553–559. https://doi.org/10.1016/j.foodcont.2008.08.003
Siragusa S, De Angelis M, Di Cagno R, Rizzello CG, Coda R, Gobbetti M (2007) Synthesis of γ -aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol 73(22):7283–7290. https://doi.org/10.1128/AEM.01064-07
Nomura M, Kimoto H, Someya Y, Suzuki I (1999) Novel characteristic for distinguishing Lactococcus lactis subsp. lactis from subsp. cremoris. Int J Syst Bacteriol 49:163–166. https://doi.org/10.1099/00207713-49-1-163
Kim SH, Shin BH, Kim YH, Nam SW, Jeon SJ (2007) Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus brevis BH2. Biotechnol Bio Process Eng 12:707–712. https://doi.org/10.1007/BF02931089
Ko CY, Lin HTV, Tsai GJ (2013) Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem 48:559–568. https://doi.org/10.1016/j.procbio.2013.02.021
Lee BJ, Kim JS, Kang YM, Lim JH, Kim YM, Lee MS, Jeong MH, Ahn CB, Je JY (2010) Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem 122:271–276. https://doi.org/10.1016/j.foodchem.2010.02.071
Villegas JM, Brown L, Savoy de Giori G, Hebert EM (2016) Optimization of batch culture conditions for GABA production by Lactobacillus brevis CRL 1942, isolated from quinoa sourdough. Food Sci Technol 67:22–26. https://doi.org/10.1016/j.lwt.2015.11.027
Md Zamri ND, Imam MU, Abd Ghafar SA, Ismail M (2014) Antioxidative effects of germinated brown rice-derived extracts on H2O2-induced oxidative stress in HepG2 Cells. Evid based complement alternat medicine 2014:1–11. https://doi.org/10.1155/2014/371907
Tajabadi N, Ebrahimpour A, Baradaran A, Rahim RA, Mahyudin NA, Abdul Manap MY, Bakar FA, Saari N (2015) Optimization of γ-aminobutyric acid production by Lactobacillus plantarum Taj-Apis362 from honeybees. Molecules 20:6654–6669. https://doi.org/10.3390/molecules20046654
FAO/WHO (2006) Probiotics in food: health and nutritional properties and guidelines for evaluation. FAO Food Nutr Pap 85. https://www.fao.org/3/a-a0512e.pdf. Accessed 12 Sep 2019
Chiang SS, Pan TM (2012) Beneficial effects of Lactobacillus paracasei subsp. Paracasei NTU 101 and its fermented products. Appl Microbiol Biotechnol 93:903–916. https://doi.org/10.1007/s00253-011-3753-x
Ouwehand AC, Salminen S, Isolauri E (2002) Probiotics: an overview of beneficial effects. Anton Van Leeuw 82: 279–289. https://doi.org/10.1023/a:1020620607611
Erkkilä S, Petäjä E (2000) Screening of commercial meat starter cultures at low pH and in the presence of bile salts for potential probiotic use. Meat Sci 55:297–300. https://doi.org/10.1016/s0309-1740(99)00156-4
Mcdonald LC, Fleming HP, Hassan HM (1990) Acid tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl Environ Microbiol 56(7):2120–2124
Akalu N, Assefa F, Dessalegn A (2017) In vitro evaluation of lactic acid bacteria isolated from traditional fermented Shamita and Kocho for their desirable characteristics as probiotics. Afr J Biotechnol 16(12):594–606. https://doi.org/10.5897/AJB2016.15307
Oh YJ, Jung DS (2015) Evaluation of probiotic properties of Lactobacillus and Pediococcus strains isolated from omegisool, a traditionally fermented millet alcoholic beverage in Korea. LWT—Food Sci Technol 63(1): 437–444. https://doi.org/10.1016/j.lwt.2015.03.005
Succi M, Tremonte P, Reale A, Sorrentino E, Grazia L, Pacifico S, Coppola R (2005) Bile salt and acid tolerance of Lactobacillus rhamnosus strains isolated from parmigiano reggiano cheese. FEMS Microbiol Lett 244:129–137. https://doi.org/10.1016/j.femsle.2005.01.037
Floros G, Hatzikamari M, Litopoulou-Tzanetaki E, Tzanetakis N (2012) Probiotic and technological properties of facultatively heterofermentative lactobacilli from Greek traditional cheeses. Food Biotechnol 26:85–105. https://doi.org/10.1080/08905436.2011.645941
Sukumar G, Ghosh AR (2010) Pediococcus spp.-a potential probiotic isolated from Khadi (an Indian fermented food) and identified by 16S rDNA sequence analysis. Afr J Food Sci 4(9):597–602
Lee H, Yoon H, Ji et al (2011) Functional properties of Lactobacillus strains isolated from kimchi. Int J Food Microbiol 145:155–161. https://doi.org/10.1016/j.ijfoodmicro.2010.12.003
Ramos CL, Thorsen L, Schwan RF, Jespersen L (2013) Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum, and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol 36:22–29. https://doi.org/10.1016/j.fm.2013.03.010
Ram C, Chander H (2003) Optimization of culture conditions of probiotic Bifidobacteria for maximal adhesion to hexadecane. World J Microbiol Biotechnol 19:407–410. https://doi.org/10.1023/a:1023946702949
Tuo Y, Yu H, Ai L, Wu Z, Guo B, Chen W (2013) Aggregation and adhesion properties of 22 Lactobacillus strains. J Dairy Sci 96:4252–4257. https://doi.org/10.3168/jds.2013-6547
De Souza BMS, Borgonovi TF, Casarotti SN et al (2018) Lactobacillus casei and Lactobacillus fermentum strains isolated from mozzarella cheese: Probiotic potential, safety, acidifying kinetic parameters and viability under gastrointestinal tract conditions. Probiotics & Antimicro Prot 11:382–396. https://doi.org/10.1007/s12602-018-9406-y
Merritt J, Niu G, Okinaga T, Qi F (2009) Autoaggregation response of Fusobacterium nucleatum. Appl Environ Microbiol 75(24):7725–7733. https://doi.org/10.1128/AEM.00916-09
Rajković J, Joković N (2015) Probiotic properties and safety assessment of lactic acid bacteria isolated from kajmak. Biol Nyssana 6(2):81–89
Vesterlund S, Vankerckhoven V, Saxelin M, Goossens H, Salminen S, Ouwehand AC (2007) Safety assessment of Lactobacillus strains: Presence of putative risk factors in faecal, blood and probiotic isolates. Int J Food Microbiol 116(3):325–331. https://doi.org/10.1016/j.ijfoodmicro.2007.02.002
Charteris WP, Kelly PM, Morelli L, Collins JK (2001) Gradient diffusion antibiotic susceptibility testing of potentially probiotic lactobacilli. J Food Prot 64(12): 2007–2014. https://doi.org/10.4315/0362-028x-64.12.2007
Zhou N, Zhang JX, Fan MT, Wang J, Guo G, Wei XY (2012) Antibiotic resistance of lactic acid bacteria isolated from Chinese yogurts. J Dairy Sci 95:4775–4783. https://doi.org/10.3168/jds.2011-5271
Mathur S, Singh R (2005) Antibiotic resistance in food lactic acid bacteria -a review. Int J Food Microbiol 105:281–295. https://doi.org/10.1016/j.ijfoodmicro.2005.03.008
Ratanaburee A, Kantachote D, Charernjiratrakul W, Sukhoom A (2013) Selection of γ-aminobutyric acid-producing lactic acid bacteria and their potential as probiotics for use as starter cultures in Thai fermented sausages (Nham). Int J Food Sci Tech 48:1371–1382. https://doi.org/10.1111/ijfs.12098
Bassyouni RH, Abdel-all WS, Abdel-all MGFS, Kamel Z (2012) Characterization of lactic acid bacteria isolated from dairy products in Egypt as a probiotic. Life Sci 9(4):2924–2933
Yuksekdag ZN, Aslim B (2010) Assessment of potential probiotic and starter properties of Pediococcus spp.isolated from turkish-type fermented sausages (sucuk). J Microbiol and Biotechnol 20:161–168 https://doi.org/10.4014/jmb.0904.04019
Salminen S, Wright AV, Morelli L et al (1998) Demonstration of safety of probiotics- a preview. Int J Food Microbiol 44:93–106. https://doi.org/10.1016/s0168-1605(98)00128-7
Funding
The second author received financial assistance from DST-WOS A(SR/WOS-A/LS-458/2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human and Animal Participants
The research does not contain any human or animal subjects for study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
BS, S., Thankappan, B., Mahendran, R. et al. Evaluation of GABA Production and Probiotic Activities of Enterococcus faecium BS5. Probiotics & Antimicro. Prot. 13, 993–1004 (2021). https://doi.org/10.1007/s12602-021-09759-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09759-7