Abstract
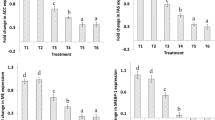
The effects of dietary Lactobacillus acidophilus (LBA) and mannan-oligosaccharide (MOS) supplementation on lipid metabolism and consequent lipid profile and health indices in broiler chicken were investigated in this study. Supplementation of 0.2% MOS along with either 106 or 107 LBA/g feed in broiler chicken downregulated hepatic expression of genes involved in lipogenesis, and upregulated expression of lipolytic genes. It caused decline of lipogenesis and increase of lipid oxidation which resulted in lower carcass fat content. None of the genes studied influenced fatty acid profile of chicken meat except the expression of stearoyl CoA (Δ9) desaturase-1 (SCD-1) whose upregulation increased monounsaturated fatty acid (MUFA) content at the cost of saturated fatty acid (SFA) content. The lipid metabolism indices of chicken meat such as ∆9 desaturase index (DI) increased in birds supplemented with 0.2% MOS along with either 106 or 107 CFU LBA/g feed, whereas no effect was observed on ∆5 + ∆6 DI. The supplementation of 0.2% MOS along with either 106 or 107 CFU LBA/g feed in birds improved the health indices of chicken meat due to upregulation of SCD-1 expression. The supplementation of 0.2% MOS along with either 106 or 107 CFU LBA/g feed in broiler chicken produced hypocholesterolemic and hypolipidemic effects with improved serum cardio-protective indices.




Similar content being viewed by others
References
Smink W, Gerrits WJ, Hovenier R, Geelen MJ, Verstegen MW, Beynen AC (2010) Effect of dietary fat sources on fatty acid deposition and lipid metabolism in broiler chickens. Poult Sci 89(11):2432–2440. https://doi.org/10.3382/ps.2010-00665
Nelson DL, Cox MM (eds) (2011) Lehninger principles of biochemistry. Worth Publishers, New York, USA
Richards MP, Poch SM, Coon CN, Rosebrough RW, Ashwell CM, McMurtry JP (2003) Feed restriction significantly alters lipogenic gene expression in broiler breeder chickens. J Nutr 133:707–715. https://doi.org/10.1093/jn/133.3.707
Tu T, Su Y, Li G, Zhang X, Tong H (2016) Expression of lipid metabolism-associated genes in male and female white feather chicken. J Poult Sci 53:118–123. https://doi.org/10.2141/jpsa.0140071
Zhao S, MA H, Zou S, Chen W, Zhao R, (2007) Hepatic lipogenesis in broiler chickens with different fat deposition during embryonic development. J Vet Med A Physiol Pathol Clin Med 54(1):1–6. https://doi.org/10.1111/j.1439-0442.2007.00898.x
Guillou H, Martin PGP, Pineau T (2008) Transcriptional regulation of hepatic fatty acid metabolism. In: Quinn PJ, Wang X (eds) Lipids in health and disease. Subcellular Biochemistry, vol 49. Springer, Dordrecht, pp 3–47. https://doi.org/10.1007/978-1-4020-8831-5_1
Jump DB (2011) Fatty acid regulation of hepatic lipid metabolism. Curr Opin Clin Nutr Metab Care 14(2):115–120. https://doi.org/10.1097/MCO.0b013e328342991c
Li H, Li Z, Liu X (2017) An overall view of the regulation of hepatic lipid metabolism in chicken revealed by new-generation sequencing. In: Manafi M (ed) Poultry Science. IntechOpen, London, UK, pp 133–147. https://doi.org/10.5772/64970
Huang JB, Zhang Y, Zhou YB, Wan XC, Zhang JS (2015) Effects of epigallocatechin gallate on lipid metabolism and its underlying molecular mechanism in broiler chickens. J Anim Physiol Anim Nutr 99:719–727. https://doi.org/10.1111/jpn.12276
Rosa F, Osorio JS, Trevisi E, Yanqui-Rivera F, Estill CT, Bionaz M (2017) 2,4-Thiazolidinedione treatment improves the innate immune response in dairy goats with induced subclinical mastitis. PPAR Res 2017:7097450.https://doi.org/10.1155/2017/7097450
Berg JM, Tymoczko JL, Stryer L (2012) Biochemistry, 7th edn. Basingstoke, WH Freeman, New York
de Souza Khatlab A, Del Vesco AP, Gasparino E, de Oliveira Neto AR (2018) Gender and age effects on the expression of genes related to lipid metabolism in broiler’s liver. Czech J Anim Sci 63:103–109.https://doi.org/10.17221/41/2017-CJAS
Basiricò L, Morera P, Lacetera N, Ronchi B, Nardone A, Bernabucci U (2011) Down-regulation of hepatic ApoB100 expression during hot season in transition dairy cows. Livest Sci 137:49–57. https://doi.org/10.1016/j.livsci.2010.09.027
Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, **ao X, Franks R, Heidenreich KA (2006) FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 281:10105–10117. https://doi.org/10.1074/jbc.M600272200
Yang X, Zhuang J, Rao K, Li X, Zhao R (2010) Effect of early feed restriction on hepatic lipid metabolism and expression of lipogenic genes in broiler chickens. Res Vet Sci 89:438–444. https://doi.org/10.1016/j.rvsc.2010.04.003
Ooi LG, Liong MT (2010) Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci 11:2499–2522. https://doi.org/10.3390/ijms11062499
Royan M, Meng GY, Othman F, Sazili AQ, Navidshad B (2011) Effects of conjugated linoleic acid, fish oil and soybean oil on PPARs (α & γ) mRNA expression in broiler chickens and their relation to body fat deposits. Int J Mol Sci 12:8581–8595. https://doi.org/10.3390/ijms12128581
Zhu K, Tan F, Mu J, Yi R, Zhou X, Zhao X (2019) Anti-obesity effects of lactobacillus fermentum CQPC05 isolated from sichuan pickle in high-fat diet-induced obese mice through PPAR-α signaling pathway. Microorganisms 7:194. https://doi.org/10.3390/microorganisms7070194
Luo H, Wang X, Chen C, Wang J, Zou X, Li C, Xu Z, Yang X, Shi W, Zeng C (2015) Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats. J Am Heart Assoc 4:e001559. https://doi.org/10.1161/JAHA.114.001559
Rather SA, Pothuraju R, Sharma RK, De S, Mir NA, Jangra S (2014) Anti-obesity effect of feeding probiotic dahi containing Lactobacillus casei NCDC 19 in high fat diet-induced obese mice. Int J Dairy Technol 67(504):509. https://doi.org/10.1111/1471-030712154
Zhang J, Zhou X, Chen B, Long X, Mu J, Pan Y, Song JL, Zhao X, Yang Z (2018) Preventive effect of Lactobacillus plantarum CQPC10 on activated carbon induced constipation in Institute of Cancer Research (ICR) mice. Appl Sci 8:1498. https://doi.org/10.3390/app8091498
Liong MT, Dunshea FR, Shah NP (2007) Effects of a synbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolemic pigs on high- and low-fat diets. Br J Nutr 98:736–744. https://doi.org/10.1017/S0007114507747803
Dev K, Mir NA, Biswas A, Kannoujia J, Begum J, Kant R, Mandal A (2020) Dietary synbiotic supplementation improves the growth performance, body antioxidant pool, serum biochemistry, meat quality, and lipid oxidative stability in broiler chickens. Anim Nutr 6:325–332. https://doi.org/10.1016/j.aninu.2020.03.002
Mir NA, Tyagi PK, Biswas AK, Tyagi PK, Mandal AB, Kumar F, Sharma D, Biswas A, Verma AK (2018) Inclusion of flaxseed, broken rice, and distillers dried grains with solubles (DDGS) in broiler chicken ration alters the fatty acid profile, oxidative stability, and other functional properties of meat. Euro J Lipid Sci Technol 120:1700470. https://doi.org/10.1002/ejlt.201700470
Bai J, Greene E, Li W, Kidd MT, Dridi S (2015) Branched-chain amino acids modulate the expression of hepatic fatty acid metabolism-related genes in female broiler chickens. Mol Nutr Food Res 59(1171):1181. https://doi.org/10.1002/mnfr.201400918
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36. https://doi.org/10.1093/nar/30.9.e36
O’Fallon JV, Busboom JR, Nelson ML, Gaskins CT (2007) A direct method for fatty acid methyl ester (FAME) synthesis: application to wet meat tissues, oils and feedstuffs. J Anim Sci 85:1511–1521. https://doi.org/10.2527/jas.2006-491
Association of Official Analytical Chemists (1995). Official methods of analysis of AOAC International.
Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K (2005) Plasma palmitoleic acid content and obesity in children. Am J Clin Nutr 82:747–750. https://doi.org/10.1093/ajcn/82.4.747
Sirri F, Castellini C, Roncarati A, Meluzzi A (2010) Effect of feeding and genotype on lipid profile of organic chicken meat. Eur J Lipid Sci Tech 112:994–1002. https://doi.org/10.1002/ejlt.200900204
Kumar F, Tyagi PK, Mir NA et al (2019) Role of flaxseed meal feeding for different durations in the lipid deposition and meat quality in broiler chickens. J Am Oil Chem Soc 96(3):261–271. https://doi.org/10.1002/aocs.12190
Pilarczyk R, Woojcik J, Sablik P, Czerniak P (2015) Fatty acid profile and health lipid indices in the raw milk of Simmental and Holstein-Friesian cows from an organic farm. S Afr J Anim Sci 45:30–38. https://doi.org/10.4314/sajas.v45i1.4
Frohlich J, Dobiášová M (2003) Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem 49:1873–1880. https://doi.org/10.1373/clinchem.2003.02255835
Saggerson D (2008) Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr 28:253–272. https://doi.org/10.1146/annurev.nutr.28.061807.155434
Khatun J, Loh TC, Akit H, Foo HL, Mohamad R (2017) Fatty acid composition, fat deposition, lipogenic gene expression and performance of broiler fed diet supplemented with different sources of oil. Anim Sci J 88(1406):1413. https://doi.org/10.1111/asj.12775
Wang Y, Zhou M, Lam KSL, Xu A (2009) Protective roles of adiponectin in obesity-related fatty liver diseases: mechanisms and therapeutic implications. Arq Bras Endocrinol Metabol 53:201–212. https://doi.org/10.1590/S0004-27302009000200012
Proszkowiec-Weglarz M, Richards MP (2009) Expression and activity of the 5’-adenosine monophosphate-activated protein kinase pathway in selected tissues during chicken embryonic development. Poult Sci 88(159):178. https://doi.org/10.3382/ps.2008-00262
Zerehdaran S, Vereijken AL, Arendonk JV, Van der Waaij EH (2005) Effect of age and housing system on genetic parameters for broiler carcass traits. Poult Sci 84(6):833–838. https://doi.org/10.1093/ps/84.6.833
Paton CM, Ntambi JM (2009) Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab 297:E28–E37. https://doi.org/10.1152/ajpendo.90897.2008
Xu H, Li X, Adams H, Kubena K, Guo S (2019) Etiology of metabolic syndrome and dietary intervention. Int J Mol Sci 20(1):128. https://doi.org/10.3390/ijms20010128
Begley M, Hill C, Gahan CGM (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72:1729–1738. https://doi.org/10.1128/AEM.72.3.1729-1738.2006
Lye HS, Rusul G, Liong MT (2010) Removal of cholesterol by Lactobacilli via incorporation of and conversion to coprostanol. J Dairy Sci 93:1383–1392. https://doi.org/10.3168/jds.2009-2574
Dikeman CL, Murphy MR, Fahey GC (2006) Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J Nutr 136:913–919. https://doi.org/10.1093/jn/136.4.913
Martarelli D, Verdenelli MC, Scuri S, Cocchioni M, Silvi S, Cecchini C, Pompei P (2011) Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr Microbiol 62:1689–1696. https://doi.org/10.1007/s00284-011-9915-3
Mikelsaar M, Zilmer M (2009) Lactobacillus fermentum ME-3–an antimicrobial and antioxidative probiotic. Microb Ecol Health Dis 21:1–27. https://doi.org/10.1080/08910600902815561
Funding
This study was funded by the Department of Biotechnology, Ministry of Science and Technology, Government of India (Grant No. BT/PR9724/AAQ/1/571/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional guidelines for the care and use of animals were followed. The experimental procedures carried out in this study were approved by the Institutional Animal Ethics Committee (IEAC) following the guidelines of “Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) 2012” established under the “Prevention of Cruelty of Animals Act 1960” of Indian Penal Code (18 September 2017/Project No. 11).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dev, K., Begum, J., Biswas, A. et al. Dietary Lactobacillus acidophilus and Mannan-Oligosaccharides Alter the Lipid Metabolism and Health Indices in Broiler Chickens. Probiotics & Antimicro. Prot. 13, 633–646 (2021). https://doi.org/10.1007/s12602-020-09717-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09717-9