Abstract
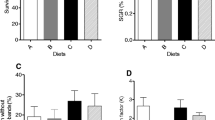
Shark eggs-based diet is the only diet by which eel larvae can grow to glass eels in captivity. However, the high level of lipids in the diet is suggested to negatively affect eel larvae. This paper examines the effect of defatted shark eggs (DSE) and hen egg yolk (HY) on growth and survival of larvae of Japanese eel Anguilla japonica. Lyophilized shark egg and commercial HY were defatted with n-hexane, and four experimental diets were prepared using both defatted and untreated shark eggs and HY. Larvae were reared for 3 weeks by feeding the experimental diets. The highest survival rate was observed in the larvae fed DSE, and larvae fed HY showed the lowest survival rate. The best growth was found in larvae fed DSE, followed by shark eggs and defatted HY, and the worst growth was in HY-fed larvae. These results show that decreasing dietary lipids improves the nutritional value of both shark eggs and HY for eel larvae. Regulation of the dietary lipid level may positively affect the larval performance of eels by combination of ingredients with a low lipid content.

Similar content being viewed by others
References
Yamamoto K, Yamauchi K (1974) Sexual maturation of Japanese eel and production of eel larvae in the aquarium. Nature 251:220–222
Tanaka H, Kagawa H, Ohta H, Okuzawa K, Hirose K (1995) The first report of eel larvae ingesting rotifers. Fish Sci 61:171–172
Tanaka H, Kagawa H, Ohta H (2001) Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture 201:51–60
Miller MJ, Chikaraishi Y, Ogawa NO, Yamada Y, Tsukamoto K, Ohkouchi N (2012) A low trophic position of Japanese eel larvae indicates feeding on marine snow. Biol Lett. doi:10.1098/rsbl.2012.0826
Tanaka H, Kagawa H, Ohta H, Unuma T, Nomura K (2003) The first production of glass eel in captivity: fish reproductive physiology: facilitates great progress in aquaculture. Fish Physiol Biochem 28:493–497
Alber M, Valiela I (1994) Biochemical composition of organic aggregates produced from marine macrophyte-derived dissolved organic matter. Limnol Oceanogr 39:717–723
Crossman DJ, Choat JH, Clements KD, Hardy T, McConchie J (2001) Detritus as food for grazing fishes on coral reefs. Limnol Oceanogr 46:1596–1605
Wilson S (2002) Nutritional value of detritus and algae in blenny territories on the Great Barrier Reef. J Exp Mar Biol Ecol 271:155–169
Masuda Y, **bo T, Imaizumi H, Furuita H, Matsunari H, Murashita K, Fujimoto H, Nagao J, Kawakami Y (2013) A step forward in development of fish protein hydrolysate-based diets for larvae of Japanese eel Anguilla japonica. Fish Sci 79:681–688
Conceição LEC, Yúfera M, Makridis P, Morais S, Dinis MT (2010) Live feeds for early stages of fish rearing. Aquac Res 41:613–640
Murashita K, Furuita H, Matsunari H, Yamamoto T, Awaji M, Nomura K, Nagao J, Tanaka H (2013) Partial characterization and ontogenetic development of pancreatic digestive enzymes in Japanese eel Anguilla japonica larvae. Fish Physiol Biochem 39:895–905
Okamura A, Yamada Y, Horie N, Mikawa N, Tanaka S, Kobayashi H, Tsukamoto K (2012) Hen egg yolk and skinned krill as possible foods for rearing leptocephalus larvae of Anguilla japonica Temminck & Schlegel. Aquac Res. doi:10.1111/j.1365-2109.2012.03160.x
Kagawa H, Tanaka H, Ohta H, Okuzawa K, Iinuma N (1997) Induced ovulation by injection of 17,20β-dihydroxy-4-pregnen-3-one in the artificially matured Japanese eel, with special reference to ovulation time. Fish Sci 63:365–637
Kagawa H, Sakurai Y, Horiuchi R, Kazeto Y, Gen K, Imaizumi H, Masuda Y (2013) Mechanism of oocyte maturation and ovulation and its application to seed production in the Japanese eel. Fish Physiol Biochem 39:13–17
Ohta H, Kagawa H, Tanaka H, Okuzawa K, Hirose K (1996) Changes in fertilization and hatching rates with time after ovulation induced by 17,20β-dihydroxy-4-pregnen-3-one in the Japanese eel, Anguilla japonica. Aquaculture 139:291–301
Hara S, Uno N, Totani Y (2007) Novel fractionation of method for soy phospholipid classes. J Fac Sci Tech Seikei Univ 44:65–73 (in Japanese with English abstract)
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Juaneda P, Rocquelin G (1985) Rapid and convenient separation of phospholipids and non phosphorous lipids from rat heart using silica cartridges. Lipid 20:40–41
Miyashita K, Inukai N, Ota T, Sasaki S, Ota T (1999) Antioxidant activity of water extracts from fish eggs on PC liposomes. Nippon Suisan Gakkaishi 65:488–494 (in Japanese with English abstract)
Furuita H, Yamamoto T, Shima T, Suzuki N, Takeuchi T (2003) Effect of arachidonic acid levels in broodstock diet on larval and egg quality of Japanese flounder Paralichthys olivaceus. Aquaculture 220:725–735
Sargent JR, McEvoy LA, Bell JG (1997) Requirements, presentation and source of polyunsaturated fatty acids in marine fish larval feeds. Aquaculture 155:117–127
Takeuchi T (1997) Essential fatty acid requirement of aquatic animals with emphasis on fish larvae and fingerlings. Rev Fish Sci 5:1–25
Izquierdo MS, Socorro J, Arantzamendi L, Hernández-Cruz CM (2000) Recent advances in lipid nutrition in fish larvae. Fish Physiol Biochem 22:97–107
Morais S, Conceição LEC, Rønnestad I, Koven W, Cahu C, Zambonino Infante JL, Dinis MT (2007) Dietary neutral lipid level and source in marine fish larvae: effects on digestive physiology and food intake. Aquaculture 268:106–122
Salhi M, Hernadez-Crus CM, Bessonart M, Izquierdo MS, Fernandez-Palacios H (1999) Effect of different dietary polar lipid levels and different n-3 HUFA content in polar lipids on gut liver histological structure of gilthead seabream Sparus aurata larvae. Aquaculture 179:253–263
Olsen AI, Attramadal Y, Reitan KI, Olsen Y (2000) Food selection and digestion characteristics of Atlantic halibut (Hippoglossus hippoglossus) larvae fed cultivated prey organisms. Aquaculture 181:293–310
Kjørsvik E, Olsen C, Wold P-A, Hoehne-Reitan K, Cahu CL, Rainuzzo J, Olsen AI, Øie G, Olsen Y (2009) Comparison of dietary phospholipids and neutral lipids on skeletal development and fatty acid composition in Atlantic cod (Gadus morhua). Aquaculture 294:246–255
Zambonino Infante JL, Cahu CL (1999) High dietary lipid levels enhance digestive tact maturation and improve Dicentrarchus labrax larval development. J Nutr 129:1195–1200
Buchet V, Zambonino Infante JL, Cahu CL (2000) Effect of lipid level in compound diet on the development of red drum (Sciaenops ocellatus) larvae. Aquaculture 184:339–347
Brinkmeyer RL, Holt GJ (1995) Response of red drum larvae to graded levels of menhaden oil in semipurified microparticulate diets. Prog Fish-Cult 57:30–36
Hamre K, Næss T, Espe M, Holm JC, Lie Ø (2001) A formulated diet for Atlantic halibut (Hippoglossus hippoglossus) larvae. Aquac Nutr 7:123–132
Robin JH, Vincent B (2003) Microparticulate diets as first food for gilthead sea bream larva (Sparus aurata): study of fatty acid incorporation. Aquaculture 225:463–474
Kvåle A, Yúfera M, Nygård E, Aursland K, Harboe T, Hamre K (2006) Leaching properties of three different mircoparticulate diets and preference of the diets in cod (Gadus morhua L.) larvae. Aquaculture 251:402–415
Morais S, Caballero MJ, Conceição LEC, Izquierdo MS, Dinis MT (2006) Dietary neutral lipid level and source in Senegalese sole (Solea senegalensis) larvae: effect on growth, lipid metabolism and digestive capacity. Comp Biochem Physiol 144B:57–69
Morais S, Torten M, Nixon O, Lutzky S, Conceição LEC, Dinis MT, Tandler A, Koven W (2006) Food intake and absorption are affected by dietary lipid level and lipid source in seabream (Sparus aurata L.) larvae. J Exp Mar Biol Ecol 331:51–63
Gawlicka A, Herold MA, Barrows FT, de la Noüe J, Hung SO (2002) Effects of dietary lipids on growth, fatty acid composition, intestinal absorption and hepatic storage in white sturgeon (Acipenser transmontanus R.) larvae. J Appl Ichthyol 18:673–681
Izquierdo MS, Tandler A, Salhi M, Kolokovski S (2001) Influence of dietary polar lipids’ quantity and quality on ingestion and assimilation of labelled fatty acids by larval gilthead seabream. Aquac Nutr 7:153–160
Kjørsvik E, Olsen Y, Rosenlund G, Vadstein O (1991) Effect of various lipid enrichments in rotifers and the development of early stages in turbot. In: Lavens P, Sorgeloos P, Jaspers E, Ollevier F (eds) Proceedings of fish and crustacean larviculture symposium. Larvi’91. European Aquaculture Society, Special Publication, vol. 15. European Aquaculture SocietyGent, Belgium, pp 20–22
Morais S, Koven W, Rønnestad I, Dinis MT, Conceição LEC (2005) Dietary protein/lipid ratio affects growth and amino acid and fatty acid absorption and metabolism in Senegalese sole (Solea senegalensis Kaup 1858) larvae. Aquaculture 246:347–357
Wold P-A, Hoehne-Reitan K, Cahu CL, Zambonino Infante J, Rainuzzo J, Kjørsvik E (2007) Phospholipids vs. neutral lipids: effects on digestive enzymes in Atlantic cod (Gadus morhua) larvae. Aquaculture 272:502–513
Gisbert E, Villeneuve L, Zambonino-Infante JL, Quazuguel P, Cahu CL (2005) Dietary phospholipids are more efficient than neutral lipids for long-chain polyunsaturated fatty acid supply in European sea bass Dicentrarchus labrax larval development. Lipids 40:609–618
Takeuchi T, Wang Q, Furuita H, Hirota T, Ishida S, Hayasawa H (2003) Development of microparticle diets for Japanese flounder Paralichthys olivaceus. Fish Sci 69:547–554
Zambonino Infante JL, Cahu CL, Peres A (1997) Partial substitution of di- and tripeptides for native proteins in sea bass diet improves Dicentrarchus labrax larval development. J Nutr 127:608–614
Cavalho AP, Sá R, Oliva-Teles A, Bergot P (2004) Solubility and peptide profile affect the utilization of dietary protein by common carp (Cyprinus carpio) during early larval stages. Aquaculture 234:319–333
Ketchen KS (1975) Age and growth of dogfish Squalus acanthias in British Columbia waters. J Fish Res Board Can 32:43–59
Sargent JR, Tocher DR, Bell JG (2002) The lipids. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic, San Diego, pp 181–257
Villeneuve L, Gisbert E, Zambonino Infante JL, Quazuguel P, Cahu CL (2005) Effect of nature of dietary lipids on European sea bass morphogenesis: implication of retinoid receptor. Br J Nutr 94:877–884
Acknowledgments
This study was partly supported by funding from the Ministry of Agriculture, Forestry and Fisheries, Government of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furuita, H., Murashita, K., Matsunari, H. et al. Decreasing dietary lipids improves larval survival and growth of Japanese eel Anguilla japonica . Fish Sci 80, 581–587 (2014). https://doi.org/10.1007/s12562-014-0713-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-014-0713-2