Abstract
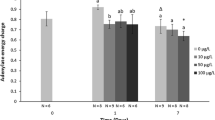
Calorie restriction (CR) in the rotifer Brachionus plicatilis extends its lifespan, as it enhances the expression of antioxidant enzymes such as manganese superoxide dismutase (Mn SOD) and catalase. Here we show that CR also increased the mRNA levels of these antioxidant enzymes upon exposure to oxidative stress. Rotifers cultured under CR showed a higher survival rate than those fed ad libitum (AL) upon exposure to 0.05–0.2 μM juglone, an oxidative stress inducer. The relative mRNA levels of Mn SOD and catalase before exposure to juglone were slightly higher in the CR rotifers than in their AL counterparts, although these differences were not statistically significant. AL rotifers showed no apparent upregulation of the mRNA levels of these antioxidant enzymes upon exposure to 0.025 and 0.05 μM juglone. In contrast, the CR rotifers increased the mRNA levels of Mn SOD and catalase by up to 5.4-fold and 4.2-fold, respectively, resulting in significant differences between their levels in AL and CR rotifers under oxidative stress conditions. Furthermore, the protein level of catalase was clearly higher in CR than in AL rotifers 6 h after exposure to oxidative stress. These results suggest that the upregulation of Mn SOD and catalase genes is involved in CR-induced resistance to oxidative stress in the rotifer.




Similar content being viewed by others
References
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Zelko IN, Mariani TJ, Folz RJ (2002) Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33:337–349
Curtis C, Landis GN, Folk D, Wehr NB, Hoe N, Waskar M, Abdueva D, Skvortsov D, Ford D, Luu A, Badrinath A, Levine RL, Bradley TJ, Tavare S, Tower J (2007) Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol 8:R262
Sun JT, Folk D, Bradley TJ, Tower J (2002) Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics 161:661–672
Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, van Remmen H, Wallace DC, Rabinovitch PS (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308:1909–1911
Koubova J, Guarente L (2003) How does calorie restriction work? Genes Dev 17:313–321
Bokov A, Chaudhuri A, Richardson A (2004) The role of oxidative damage and stress in aging. Mech Ageing Dev 125:811–826
Yen K, Patel HB, Lublin AL, Mobbs CV (2009) SOD isoforms play no role in lifespan in ad lib or dietary restricted conditions, but mutational inactivation of SOD-1 reduces life extension by cold. Mech Ageing Dev 130:173–178
Rohrdanz E, Schmuck G, Ohler S, Kahl R (2001) The influence of oxidative stress on catalase and MnSOD gene transcription in astrocytes. Brain Res 900:128–136
Kemp TJ, Causton HC, Clerk A (2003) Changes in gene expression induced by H2O2 in cardiac myocytes. Biochem Biophys Res Commun 307:416–421
Park SK, Tedesco PM, Johnson TE (2009) Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell 8:258–269
Wheelock CE, Baumgartner TA, Newman JW, Wolfe MF, Tjeerdema RS (2002) Effect of nutritional state on Hsp60 levels in the rotifer Brachionus plicatilis following toxicant exposure. Aquat Toxicol 61:89–93
Yoshinaga T, Kaneko G, Kinoshita S, Tsukamoto K, Watabe S (2003) The molecular mechanisms of life history alterations in a rotifer: a novel approach in population dynamics. Comp Biochem Physiol B 136:715–722
Oo AKS, Kaneko G, Hirayama M, Kinoshita S, Watabe S (2010) Identification of genes differentially expressed by calorie restriction in the rotifer (Brachionus plicatilis). J Comp Physiol B 180:105–116
Krall J, Bagley AC, Mullenbach GT, Hallewell RA, Lynch RE (1988) Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J Biol Chem 263:1910–1914
Kaneko G, Yoshinaga T, Yanagawa Y, Ozaki Y, Tsukamoto K, Watabe S (2011) Calorie restriction-induced maternal longevity is transmitted to their daughters in a rotifer. Funct Ecol 25:209–216
Tanaka C, Hashimoto Y, Nakao S, Yoshinaga T (2009) Effect of juglone on the survival time of two Brachionus species (Rotifera): species-specific tolerance against oxidative stress. Fish Sci 75:191–194
Yoshinaga T, Minegishi Y, Rumengan IFM, Kaneko G, Furukawa S, Yanagawa Y, Tsukamoto K, Watabe S (2004) Molecular phylogeny of the rotifers with two Indonesian Brachionus linages. Coast Mar Sci 29:45–56
Ozaki Y, Kaneko G, Yanagawa Y, Watabe S (2010) Calorie restriction in the rotifer Brachionus plicatilis enhances hypoxia tolerance in association with the increased mRNA levels of glycolytic enzymes. Hydrobiologia 649:267–277
Kaneko G, Yoshinaga T, Yanagawa Y, Kinoshita S, Tsukamoto K, Watabe S (2005) Molecular characterization of Mn-superoxide dismutase and gene expression studies in dietary restricted Brachionus plicatilis rotifers. Hydrobiologia 546:117–123
Takabe W, Li RS, Ai LS, Yu F, Berliner JA, Hsiai TK (2010) Oxidized low-density lipoprotein-activated c-Jun NH2-terminal kinase regulates manganese superoxide dismutase ubiquitination. Arterioscler Thromb Vasc Biol 30:436–441
Cao C, Leng YM, Liu X, Yi YP, Li P, Kufe D (2003) Catalase is regulated by ubiquitination and proteosomal degradation. Role of the c-Abl and Arg tyrosine kinases. Biochemistry 42:10348–10353
Snell TW, Joaquim-Justo C (2007) Workshop on rotifers in ecotoxicology. Hydrobiologia 593:227–232
Cochrane BJ, Irby RB, Snell TW (1991) Effects of copper and tributyltin on stress protein abundance in the rotifer Brachionus-plicatilis. Comp Biochem Physiol C 98:385–390
Cochrane BJ, Mattley YD, Snell TW (1994) Polymerase chain-reaction as a tool for develo** stress protein probes. Environ Toxicol Chem 13:1221–1229
Wheelock CE, Wolfe MF, Olsen H, Tjeerdema RS, Sowby ML (1999) Hsp60-induced tolerance in the rotifer Brachionus plicatilis exposed to multiple environmental contaminants. Arch Environ Contam Toxicol 36:281–287
Roberts MH, Sved DW, Felton SP (1987) Temporal changes in AHH and SOD activities in feral spot from the Elizabeth River, a polluted subestuary. Mar Environ Res 23:89–101
Winston GW (1991) Oxidants and antioxidants in aquatic animals. Comp Biochem Physiol C 100:173–176
Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424:277–284
Henderson ST, Johnson TE (2001) daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol 11:1975–1980
Huang HJ, Tindall DJ (2007) Dynamic FoxO transcription factors. J Cell Sci 120:2479–2487
Suga K, Mark Welch D, Tanaka Y, Sakakura Y, Hagiwara A (2007) Analysis of expressed sequence tags of the cyclically parthenogenetic rotifer Brachionus plicatilis. PLoS ONE 2:e671
Denekamp NY, Thorne MAS, Clark MS, Kube M, Reinhardt R, Lubzens E (2009) Discovering genes associated with dormancy in the monogonont rotifer Brachionus plicatilis. BMC Genomics 10:108
Yoshinaga T, Kaneko G, Kinoshita S, Furukawa S, Tsukamoto K, Watabe S (2005) Insulin-like growth factor signaling pathway involved in regulating longevity of rotifers. Hydrobiologia 546:347–352
Acknowledgments
We express sincere thanks to Professor A. Hagiwara, Nagasaki University, for providing the Ishikawa strain of Brachionus plicatilis. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Y. Ozaki was supported by a research fellowships from the Japan Society for the Promotion of Science. M. Kailasam thanks the Department of Biotechnology, Ministry of Science and Technology, Government of India for awarding him a DBT Overseas Associateship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kailasam, M., Kaneko, G., Oo, A.K.S. et al. Effects of calorie restriction on the expression of manganese superoxide dismutase and catalase under oxidative stress conditions in the rotifer Brachionus plicatilis . Fish Sci 77, 403–409 (2011). https://doi.org/10.1007/s12562-011-0334-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-011-0334-y