Abstract
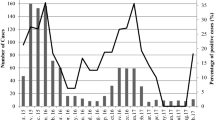
Environmental approach has proven to be a useful tool for epidemiological studies demonstrating through environmental studies the diversity of viruses circulating in a given population. The aim of this study was to perform a phylogenetic characterization of the group A rotavirus (RVA) glycoprotein (G)- and protease-sensitive (P)-genotypes obtained from sewage samples (n = 116) collected in six cities of Uruguay during March 2011 to April 2013. A worldwide standardized semi-nested multiplex RT-PCR (SNM RT-PCR) protocol directed against VP4 and VP7 genes were conducted for RVA detection and consensual DNA fragments were submitted to nucleotide sequencing. P and/or G genotype was successfully determined by phylogenetic analysis in 61 % (n = 37) of the positive samples obtained by SNM RT-PCR (n = 61). The RVA genotypes were as follow: G1 (n = 2), G2 (n = 14), G3 (n = 5), G12 (n = 2), P[4] (n = 4), P[8] (n = 16), and P[3] (n = 2). Interestingly, through phylogenetic analysis, emerging, and uncommon human genotypes could be detected. Results obtained from the comparison of RVA genotypes detected in the current study and Uruguayan RVA strains previously described for contemporary clinical pediatric cases showed that monitoring sewage may be a good screening option for a rapid and economical overview of the circulating genotypes in the surrounding human population and a useful approximation to study RVA epidemiology in a future vaccine monitoring program. The present study represents the first report in Uruguay that describes the phylogenetic diversity of RVA from urban sewage samples.




Similar content being viewed by others
References
Arista, S., Giammanco, G. M., De Grazia, S., Ramirez, S., Lo Biundo, C., Colomba, C., et al. (2006). Heterogeneity and temporal dynamics of evolution of G1 human rotaviruses in a settled population. Journal of Virology, 80, 10724–10733.
Banerjee, I., Iturriza-Gomara, M., Rajendran, P., Primrose, B., Ramani, S., Gray, J. J., et al. (2007). Molecular characterization of G11P[25] and G3P[3] human rotavirus strains associated with asymptomatic infection in South India. Journal of Medical Virology, 79(11), 1768–1774.
Bányai, K., László, B., Duque, J., Steele, A. D., Nelson, E. A., Gentsch, J. R., et al. (2012). Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: Insights for understanding the impact of rotavirus vaccination programs. Vaccine, 30(1), A122–A130.
Berois, M., Libersou, S., Russi, J., Arbiza, J., & Cohen, J. (2003). Genetic variation in the VP7 gene of human rotavirus isolated in Montevideo–Uruguay from 1996–1999. Journal of Medical Virology, 71(3), 456–462.
Bosch, A., Guix, S., Sano, D., & Pintó, R. M. (2008). New tools for the study and direct surveillance of viral pathogens in water. Current Opinion in Biotechnology, 19(3), 295–301.
Bucardo, F., Lindgren, P. E., Svensson, L., & Nordgren, J. (2011). Low prevalence of rotavirus and high prevalence of norovirus in hospital and community wastewater after introduction of rotavirus vaccine in Nicaragua. PLoS One, 6(10), e25962.
Carvalho-Costa, F. A., de Mello Volotão, E., de Assis, R. M., Fialho, A. M., de Andrade, J. D. S., Rocha, L. N., et al. (2011). Laboratory-based rotavirus surveillance during the introduction of a vaccination program, Brazil, 2005–2009. The Pediatric Infectious Disease Journal, 30(1), S35–S41.
Castello, A. A., Argüelles, M. H., Rota, R. P., Olthoff, A., Jiang, B., Glass, R. I., et al. (2006). Molecular epidemiology of group A rotavirus diarrhea among children in Buenos Aires, Argentina, from 1999 to 2003 and emergence of the infrequent genotype G12. Journal of Clinical Microbiology, 44(6), 2046–2050.
Cook, S. M., Glass, R. I., LeBaron, C. W., & Ho, M. S. (1990). Global seasonality of rotavirus infections. Bulletin of the World Health Organization, 68(2), 171–177.
Das, B. K., Gentsch, J. R., Cicirello, H. G., Woods, P. A., Gupta, A., Ramachandran, M., et al. (1994). Characterization of rotavirus strains from newborns in New Delhi, India. Journal of Clinical Microbiology, 32, 1820–1822.
Espinola, E. E., Parra, G. I., Russomando, G., & Arbiza, J. (2008). Genetic diversity of the VP4 and VP7 genes affects the genoty** of rotaviruses: Analysis of Paraguayan strains. Infection, Genetics and Evolution, 8(1), 94–99.
Espinosa, A. C., Mazari-Hiriart, M., Espinosa, R., Maruri-Avidal, L., Méndez, E., & Arias, C. F. (2008). Infectivity and genome persistence of rotavirus and astrovirus in groundwater and surface water. Water Research, 42(10–11), 2618–2628.
Esteban, L. E., Rota, R. P., Gentsch, J. R., Jiang, B., Esona, M., Glass, R. I., et al. (2010). Molecular epidemiology of group A rotavirus in Buenos Aires, Argentina 2004–2007: Reemergence of G2P[4] and emergence of G9P[8] strains. Journal of Medical Virology, 82(6), 1083–1093.
Estes, M., & Greenberg, H. (2013). Rotaviruses. In D. M. Knipe, P. M. Howley, J. I. Cohen, D. E. Griffin, R. A. Lamb, M. A. Martin, et al. (Eds.), Fields virology (6th ed.). Philadelphia: Wolters Kluwer business/Lippincott Williams and Wilkins.
Fumian, T. M., Leite, J. P., Castello, A. A., Gaggero, A., Caillou, M. S., & Miagostovich, M. P. (2010). Detection of rotavirus A in sewage samples using multiplex qPCR and an evaluation of the ultracentrifugation and adsorption-elution methods for virus concentration. Journal of Virological Methods, 170(1–2), 42–46.
Fumian, T. M., Leite, J. P. G., Rose, T. L., Prado, T., & Miagostovich, M. P. (2011). One year environmental surveillance of rotavirus specie A (RVA) genotypes in circulation after the introduction of the Rotarix vaccine in Rio de Janeiro, Brazil. Water Research, 45, 5755–5763.
Gentsch, J. R., Glass, R. I., Woods, P., Gouvea, V., Gorziglia, M., Flores, J., et al. (1992). Identification of group A rotavirus gene 4 types by polymerase chain reaction. Journal of Clinical Microbiology, 30, 1365–1373.
Gómara, M. I., Cubitt, D., Desselberger, U., & Gray, J. (2001). Amino acid substitution within the VP7 protein of G2 rotavirus strains associated with failure to serotype. Journal of Clinical Microbiology, 39(10), 3796–3798.
Gómez, M. M., Carvalho-Costa, F. A., de Mello Volotão, E., Rose, T. L., da Silva, M. F., Fialho, A. M., et al. (2014a). Prevalence and genomic characterization of G2P[4] group A rotavirus strains during monovalent vaccine introduction in Brazil. Infection, Genetics and Evolution, 28, 486–494.
Gómez, M. M., Carvalho-Costa, F. A., de Mello Volotão, E., Rose, T. L., da Silva, M. F., Fialho, A. M., et al. (2014b). A decade of G3P[8] and G9P[8] rotaviruses in Brazil: Epidemiology and evolutionary analyses. Infection, Genetics and Evolution, 28, 389–397.
Gómez, M. M., Resque, H. R., de Mello Volotão, E., Rose, T. L., da Silva, M. F. M., Heylen, E., et al. (2014c). Distinct evolutionary origins of G12P[8] and G12P[9] group A rotavirus strains circulating in Brazil. Infection, Genetics and Evolution, 28, 385–388.
Graham, D. Y., Dufour, G. R., & Estes, M. K. (1987). Minimal infective dose of rotavirus. Archives of Virology, 92, 261–271.
Hopkins, R. S., Gaspard, G. B., Williams, F. P, Jr, Karlin, R. J., Cukor, G., & Blacklow, N. R. (1984). A community waterborne gastroenteritis outbreak: Evidence for rotavirus as the agent. American Journal of Public Health, 74(3), 263–265.
I.N.E. (2011). Uruguayan National Institute of Statistic. http://www.ine.gub.uy/censos2011/index.html (Spanish). Accessed 11 Nov 2014.
Kamel, A. H., Ali, M. A., El-Nady, H. G., Aho, S., Pothier, P., & Belliot, G. (2010). Evidence of the co-circulation of enteric viruses in sewage and in the population of Greater Cairo. Journal of Applied Microbiology, 108(5), 1620–1629.
Li, D., Gu, A. Z., Zeng, S. Y., Yang, W., He, M., & Shi, H. C. (2011). Monitoring and evaluation of infectious rotaviruses in various wastewater effluents and receiving waters revealed correlation and seasonal pattern of occurrences. Journal of Applied Microbiology, 110(5), 1129–1137.
Linhares, A. C., Stupka, J. A., Ciapponi, A., Bardach, A. E., Glujovsky, D., Aruj, P. K., et al. (2011). Burden and ty** of rotavirus group A in Latin America and the Caribbean: Systematic review and meta-analysis. Reviews in Medical Virology, 21(2), 89–109.
Lizasoain, A., Tort, L. F. L., García, M., Gómez, M. M., Leite, J. P. G., Miagostovich, M. P., et al. (2015). Sewage surveillance reveals the presence of canine GVII norovirus and canine astrovirus in Uruguay. Archives of Virology. doi:10.1007/s00705-015-2571-3.
Luchs, A., Cilli, A., Morillo, S. G., de Carmona, R. C., & Timenetsky, M. D. C. (2012). Rare G3P[3] rotavirus strain detected in Brazil: Possible human-canine interspecies transmission. Journal of Clinical Virology, 54(1), 89–92.
Mandile, M. G., Esteban, L. E., Argüelles, M. H., Mistchenko, A., Glikmann, G., & Castello, A. A. (2014). Surveillance of group A Rotavirus in Buenos Aires 2008-2011, long lasting circulation of G2P[4] strains possibly linked to massive monovalent vaccination in the region. Journal of Clinical Virology, 60(3), 282–289.
Martínez, M., Amarilla, A. A., Galeano, M. E., Aquino, V. H., Fariña, N., Russomando, G., et al. (2010). Predominance of rotavirus G2P[4] and emergence of G12P[9] strains in Asunción, Paraguay, 2006–2007. Archives of Virology, 155(4), 525–533.
Matthijnssens, J., Ciarlet, M., McDonald, S. M., Attoui, H., Bányai, K., Brister, J. R., et al. (2011a). Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Archives of Virology, 156, 1397–1413.
Matthijnssens, J., De Grazia, S., Piessens, J., Heylen, E., Zeller, M., Giammanco, G. M., et al. (2011b). Multiple reassortment and interspecies transmission events contribute to the diversity of feline, canine and feline/canine-like human group A rotavirus strains. Infection, Genetics and Evolution, 11(6), 1396–1406.
Matthijnssens, J., Heylen, E., Zeller, M., Rahman, M., Lemey, P., & Van Ranst, M. (2010). Phylodynamic analyses of rotavirus genotypes G9 and G12 underscore their potential for swift global spread. Molecular Biology and Evolution, 27(10), 2431–2436.
Matthijnssens, J., & Van Ranst, M. (2012). Genotype constellation and evolution of group A rotaviruses infecting humans. Current Opinion in Virology, 2(4), 426–433.
Metcalf, T. G., Melnick, J. L., & Estes, M. K. (1995). Environmental virology: From detection of virus in sewage and water by isolation to identification by molecular biology–a trip of over 50 years. Annual Review of Microbiology, 49, 461–487.
Page, N. A., & Steele, A. D. (2004). Antigenic and genetic characterization of serotype G2 human rotavirus strains from South Africa from 1984 to 1998. Journal of Medical Virology, 72(2), 320–327.
Pina, S., Jofre, J., Emerson, S. U., Purcell, R. H., & Girones, R. (1998). Characterization of a strain of infectious hepatitis E virus isolated from sewage in an area where hepatitis E is not endemic. Applied and Environmental Microbiology, 64(11), 4485–4488.
Posada, D. (2008). jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution, 25(7), 1253–1256.
Prado, T., & Miagostovich, M. P. (2014). Environmental virology and sanitation in Brazil: A narrative review. Cadernos de Saúde Pública, 30(7), 1367–1378.
Rajal, V. B., McSwain, B. S., Thompson, D. E., Leutenegger, C. M., Kildare, B. J., & Wuertz, S. (2007). Validation of hollow fiber ultrafiltration and real-time PCR using bacteriophage PP7 as surrogate for the quantification of viruses from water samples. Water Research, 41(7), 1411–1422.
Rodriguez-Diaz, J., Querales, L., Caraballo, L., Vizzi, E., Liprandi, F., Takiff, H., et al. (2009). Detection and characterization of waterborne gastroenteritis viruses in urban sewage and sewage-polluted river waters in Caracas, Venezuela. Applied and Environmental Microbiology, 75(2), 387–394.
Ruggeri, F. M., Bonomo, P., Ianiro, G., Battistone, A., Delogu, R., Germinario, C., et al. (2015). Rotavirus genotypes in sewage treatment plants and in children hospitalized with acute Diarrhea in Italy, 2010–2011. Applied and Environmental Microbiology, 81(1), 241–249.
Ruiz-Palacios, G. M., Pérez-Schael, I., Velázquez, F. R., Abate, H., Breuer, T., Clemens, S. C., et al. (2006). Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. The New England Journal of Medicine, 354(1), 11–22.
Santos, N., & Hoshino, Y. (2005). Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Reviews in Medical Virology, 15(1), 29–56.
Simmonds, M. K., Armah, G., Asmah, R., Banerjee, I., Damanka, S., Esona, M., et al. (2008). New oligonucleotide primers for P-ty** of rotavirus strains: Strategies for ty** previously untypeable strains. Journal of Clinical Virology, 42(4), 368–373.
Steinmann, J. (1981). Detection of rotavirus in sewage. Applied and Environmental Microbiology, 41(4), 1043–1045.
Stupka, J. A., Degiuseppe, J. I., Parra, G. I., & Network, Argentinean National Rotavirus Surveillance. (2012). Increased frequency of rotavirus G3P[8] and G12P[8] in Argentina during 2008–2009: Whole-genome characterization of emerging G12P[8] strains. Journal of Clinical Virology, 54(2), 162–167.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10), 2731–2739.
Thongprachum, A., Chan-it, W., Khamrin, P., Okitsu, S., Nishimura, S., Kikuta, H., et al. (2013). Reemergence of new variant G3 rotavirus in Japanese pediatric patients, 2009-2011. Infection, Genetics and Evolution, 13, 168–174.
Tort, L. F. L., Victoria, M., Lizasoain, A. A., Castells, M., Maya, L., Gómez, M. M., et al. (2015). Molecular epidemiology of group a rotavirus among children admitted to hospital in Salto, Uruguay, 2011–2012: First detection of the emerging genotype G12. Journal of Medical Virology, 87(5), 754–763.
Trojnar, E., Sachsenröder, J., Twardziok, S., Reetz, J., Otto, P. H., & Johne, R. (2013). Identification of an avian group A rotavirus containing a novel VP4 gene with a close relationship to those of mammalian rotaviruses. Journal of General Virology, 94, 136–142.
Vesikari, T., Matson, D. O., Dennehy, P., Van Damme, P., Santosham, M., Rodriguez, Z., et al. (2006). Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. The New England Journal of Medicine, 354(1), 23–33.
Victoria, M., Tort, L. F. L., García, M., Lizasoain, A., Maya, L., Leite, J. P. G., et al. (2014). Assessment of gastroenteric viruses from wastewater directly discharged into Uruguay River, Uruguay. Food and Environmental Virology, 6(2), 116–124.
Villena, C., Gabrieli, R., Pintó, R. M., Guix, S., Donia, D., Buonomo, E., et al. (2003). A large infantile gastroenteritis outbreak in Albania caused by multiple emerging rotavirus genotypes. Epidemiology and Infection, 131(3), 1105–1110.
Walker, C. L., Rudan, I., Liu, L., Nair, H., Theodoratou, E., Bhutta, Z. A., et al. (2013). Global burden of childhood pneumonia and diarrhoea. Lancet, 381(9875), 1405–1416.
WHO. (2009). Manual of rotavirus detection and characterization methods. Immunization, Vaccines and Biologicals, WHO/IVB/08.17.
Acknowledgments
We want to thank the financial support by the program ‘‘Polo de Desarrollo Universitario’’ (PDU), Universidad de la República (UdelaR), Uruguay; Project PCPP 023/2011 of ‘‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior’’ (CAPES, Brazil); ‘‘Agencia Nacional de Investigación e Innovación’’ project ANII-ALI-2009-1-1603; and project CSIC I+D 2010, Universidad de la República (UdelaR). We also thank “Sección Bioquímica, Instituto de Biología, Facultad de Ciencias” and “Instituto Pasteur, Montevideo, Uruguay”, for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12560_2015_9213_MOESM1_ESM.pdf
Supplementary material 1 (PDF 197 kb). Online Resource 1 Table of primers used for G/P detection and ty** of group A rotavirus (RVA) by semi-nested multiplex RT-PCR and phylogenetic analysis
12560_2015_9213_MOESM2_ESM.pdf
Supplementary material 2 (PDF 265 kb). Online Resource 2 Table of sewage samples analyzed in this study with their respective G- and/or P-types types determined by phylogenetic studies
12560_2015_9213_MOESM3_ESM.tif
Supplementary material 3 (TIFF 3239 kb). Online Resource 3 G- and P-types distribution of group A rotavirus (RVA) detected according to the collection sites in Northwestern -a) to d)- and Eastern -e) and f)- regions of Uruguay
12560_2015_9213_MOESM4_ESM.tif
Supplementary material 4 (TIFF 3013 kb). Online Resource 4 Maximum likelihood phylogenetic tree analysis of the G3 group A rotavirus (RVA) strains detected in sewage from Uruguay. Strains in the trees are shown by name following the RV strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Prototype strains previously described are also shown by accession numbers. Strains isolated in this study at the Northwestern region of Uruguay are marked with a filled square. Strains isolated in this study at the Eastern region of Uruguay are marked with a filled triangle. Uruguayan strains previously described that were detected from clinical samples collected from hospitalized children with acute diarrhea in Salto city, Uruguay, in 2011 and 2012, are marked with a filled circle. The trees were constructed with Tamura 3-parameter+G+I model as determined by using the jModelTest program. Numbers at the internal nodes in the tree indicate Bootstrap values (only values above 70 % are shown). The scale bar at the bottom represents substitutions per nucleotide position (nt.subst./site)
Rights and permissions
About this article
Cite this article
Tort, L.F.L., Victoria, M., Lizasoain, A. et al. Detection of Common, Emerging and Uncommon VP4, and VP7 Human Group A Rotavirus Genotypes from Urban Sewage Samples in Uruguay. Food Environ Virol 7, 342–353 (2015). https://doi.org/10.1007/s12560-015-9213-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-015-9213-5