Abstract
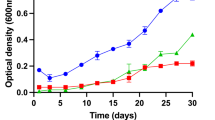
During a study of the marine actinobacterial biodiversity, a large number of Brevibacterium strains were isolated. Of these, five that have relatively low 16S rRNA gene similarity (98.5–99.3%) with validly published Brevibacterium species, were chosen to determine taxonomic positions. On the basis of 16S rRNA gene sequence analysis and BOX-PCR fingerprinting, strains o2T, YB235T, and WO024T were selected as representative strains. Genomic analyses, including average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH), clearly differentiated the three strains from each other and from their closest relatives, with values ranging from 82.8% to 91.5% for ANI and from 26.7% to 46.5% for dDDH that below the threshold for species delineation. Strains YB235T, WO024T, and o2T all exhibited strong and efficient decolorization activity in congo red (CR) dyes, moderate decolorization activity in toluidine blue (TB) dyes and poor decolorization in reactive blue (RB) dyes. Genes coding for peroxidases and laccases were identified and accounted for these strains’ ability to effectively oxidize a variety of dyes with different chemical structures. Mining of the whole genome for secondary metabolite biosynthesis gene clusters revealed the presence of gene clusters encoding for bacteriocin, ectoine, NRPS, siderophore, T3PKS, terpene, and thiopeptide. Based on the phylogenetic, genotypic and phenotypic data, strains o2T, YB235T and WO024T clearly represent three novel taxa within the genus Brevibacterium, for which the names Brevibacterium limosum sp. nov. (type strain o2T = JCM 33844T = MCCC 1A09961T), Brevibacterium pigmenatum sp. nov. (type strain YB235T = JCM 33843T = MCCC 1A09842T) and Brevibacterium atlanticum sp. nov. (type strain WO024T = JCM 33846T = MCCC 1A16743T) are proposed.
Similar content being viewed by others
Change history
01 November 2021
An Erratum to this paper has been published: https://doi.org/10.1007/s12275-021-0235-4
References
Anast, J.M., Dzieciol, M., Schultz, D.L., Wagner, M., Mann, E., and Schmitz-Esser, S. 2019. Brevibacterium from Austrian hard cheese harbor a putative histamine catabolism pathway and a plasmid for adaptation to the cheese environment. Sci. Rep.9, 6164.
Arndt, D., Grant, J.R., Marcu, A., Sajed, T., Pon, A., Liang, Y., and Wishart, D.S. 2016. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res.44, W16–W21.
Aziz, R.K., Bartels, D., Best, A.A., DeJongh, M., Disz, T., Edwards, R.A., Formsma, K., Gerdes, S., Glass, E.M., Kubal, M., et al. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics9, 75.
Benjamin, L., Vancanneyt, M., Dawyndt, P., Cnockaert, M., Zhang, J., Huang, Y., Liu, Z., and Swings, J. 2004. BOX-PCR fingerprinting as a powerful tool to reveal synonymous names in the genus Streptomyces. emended descriptions are proposed for the species Streptomyces cinereorectus, S. fradiae, S. tricolor, S. colombiensis, S. filamentosus, S. vinaceus and S. phaeopurpureus. Syst. Appl. Microbiol.27, 84–92.
Bertelli, C., Laird, M.R., Williams, K.P., Simon Fraser University Research Computing Group, Lau, B.Y., Hoad, G., Winsor, G.L., and Brinkman, F.S.L. 2017. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res.45, W30–W35.
Bhadra, B., Raghukumar, C., Pindi, P.K., and Shivaji, S. 2008. Brevibacterium oceani sp. nov., isolated from deep-sea sediment of the Chagos Trench, Indian Ocean. Int. J. Syst. Evol. Microbiol.58, 57–60.
Blin, K., Shaw, S., Steinke, K., Villebro, R., Ziemert, N., Lee, S.Y., Medema, M.H., and Weber, T. 2019. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res.47, W81–W87.
Bonnarme, P., Psoni, L., and Spinnler, H.E. 2000. Diversity of L-methionine catabolism pathways in cheese-ripening bacteria. Appl. Environ. Microbiol.66, 5514–5517.
Breed, R.S. 1953. The families developed from Bacteriaceae Cohn with a description of the family Brevibacteriaceae. Riass. Commun. VI Congr. Int. Microbiol. Roma1, 10–15.
Cerny, G. 1978. Studies on the aminopeptidase test for the distinction of Gram-negative from Gram-positive bacteria. European J. Appl. Microbiol. Biotechnol.5, 113–122.
Chatterjee, P., Samaddar, S., Niinemets, U., and Sa, T.M. 2018. Brevibacterium linens RS16 confers salt tolerance to Oryza sativa genotypes by regulating antioxidant defense and H+ ATPase activity. Microbiol. Res.215, 89–101.
Chen, P., Zhang, L., Wang, J., Ruan, J., and Huang, Y. 2016. Brevibacterium sediminis sp. nov., isolated from deep-sea sediments from the Carlsberg and Southwest Indian Ridges. Int. J. Syst. Evol. Microbiol.66, 5268–5274.
Chin, C.S., Alexander, D.H., Marks, P., Klammer, A.A., Drake, J., Heiner, C., Clum, A., Copeland, A., Huddleston, J., Eichler, E.E., et al. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods10, 563–569.
Choi, E.J., Lee, S.H., Jung, J.Y., and Jeon, C.O. 2013. Brevibacterium jeotgali sp. nov., isolated from jeotgal, a traditional Korean fermented seafood. Int. J. Syst. Evol. Microbiol.63, 3430–3436.
Collins, M.D., Farrow, J.A.E., Goodfellow, M., and Minnikin, D.E. 1983. Brevibacterium casei sp. nov. and Brevibacterium epidermidis sp. nov. Syst. Appl. Microbiol.4, 388–395.
Collins, M.D., Jones, D., Keddie, R.M., and Sneath, P.H.A. 1980. Reclassification of Chromobacterium iodinum (Davis) in a redefined genus Brevibacterium (Breed) as Brevibacterium iodinum nom.rev.; comb.nov. Microbiology120, 1–10.
Couvin, D., Bernheim, A., Toffano-Nioche, C., Touchon, M., Michalik, J., Néron, B., Rocha, E.P.C., Vergnaud, G., Gautheret, D., and Pourcel, C. 2018. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res.46, W246–W251.
Díez-Mendez, A., García-Fraile, P., Solano, F., and Rivas, R. 2019. The ant Lasius niger is a new source of bacterial enzymes with biotechnological potential for bleaching dye. Sci. Rep.9, 15217.
Felsenstein, J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol.17, 368–376.
Fitch, W.M. 1971. Toward defining the course of evolution: minimum change for a specific tree topology. Syst. Zool.20, 406–416.
Forgacs, E., Cserháti, T., and Oros, G. 2004. Removal of synthetic dyes from wastewaters: a review. Environ. Int.30, 953–971.
Gavrish, E.Y., Krauzova, V.I., Potekhina, N.V., Karasev, S.G., Plotnikova, E.G., Altyntseva, O.V., Korosteleva, L.A., and Evtushenko, L.I. 2004. Three new species of Brevibacteria, Brevibacterium antiquum sp. nov., Brevibacterium aurantiacum sp. nov., and Brevibacterium permense sp. nov. Microbiology73, 176–183.
Goodfellow, M., Kämpfer, P., Busse, H.J., Trujillo, M.E., Suzuki, K., Ludwig, W., and Whitman, W.B. 2012. Bergey’s Manual of Systematic Bacteriology: Volume 5: The Actinobacteria. Springer, New York, USA.
Goris, J., Konstantinidis, K.T., Klappenbach, J.A., Coenye, T., Vandamme, P., and Tiedje, J.M. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol.57, 81–91.
Heyrman, J., Verbeeren, J., Schumann, P., Devos, J., Swings, J., and De Vos, P. 2004. Brevibacterium picturae sp. nov., isolated from a damaged mural painting at the Saint-Catherine chapel (Castle Herberstein, Austria). Int. J. Syst. Evol. Microbiol.54, 1537–1541.
Hyatt, D., Chen, G.L., LoCascio, P.F., Land, M.L., Larimer, F.W., and Hauser, L.J. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics11, 119.
Ivanova, P.E., Christen, R., Alexeeva, Y.V., Zhukova, N.V., Gorshkove, N.M., Lysenko, A.M., Mikhailov, V.V., and Nicolau, D.V. 2004. Brevibacterium celere sp. nov., isolated from degraded thallus of a brown alga. Int. J. Syst. Evol. Microbiol.54, 2107–2111.
Kaiser, P., Geyer, R., Surmann, P., and Fuhrmann, H. 2012. LC-MS method for screening unknown microbial carotenoids and isoprenoid quinones. J. Microbiol. Methods88, 28–34.
Kanehisa, M., Sato, Y., and Morishima, K. 2016. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol.428, 726–731.
Komagata, K. and Iizuka, H. 1964. New species of Brevibacterium isolated from rice. Nippon Nōgeikagaku Kaishi38, 496–502.
Kumar, A., İnce, İ.A., Katı, A., and Chakraborty, R. 2013. Brevibacterium siliguriense sp. nov., a facultatively oligotrophic bacterium isolated from river water. Int. J. Syst. Evol. Microbiol.63, 511–515.
Kumar, S., Stecher, G., and Tamura, K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol.33, 1870–1874.
Lagesen, K., Hallin, P., Rødland, E.A., Staerfeldt, H.H., Rognes, T., and Ussery, D.W. 2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res.35, 3100–3108.
Lane, D.J. 1991. 16S/23S rRNA sequencing. In Stackebrandt, E. and Goodfellow, M. (eds.), Nucleic Acid Techniques in Bacterial Systematic, pp. 115–175. John Wiley and Sons, New York, USA.
Lechevalier, M.P. and Lechevalier, H. 1970. Chemical composition as a criterion in the classification of aerobic actinomycetes. Int. J. Syst. Bacteriol.20, 435–443.
Li, W.J., Xu, P., Schumann, P., Zhang, Y.Q., Pukall, R., Xu, L.H., Stackebrandt, E., and Jiang, C.L. 2007. Georgenia ruanii sp. nov., a novel actinobacterium isolated from forest soil in Yunnan (China), and emended description of the genus Georgenia. Int. J. Syst. Evol. Microbiol.57, 1424–1428.
Lim, S.H., Lee, Y.H., and Kang, H.W. 2013. Efficient recovery of lignocellulolytic enzymes of spent mushroom compost from oyster mushrooms, Pleurotus spp., and potential use in dye decolorization. Mycobiology41, 214–220.
Lowe, T.M. and Chan, P.P. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res.44, 54–57.
McMullan, G., Meehan, C., Conneely, A., Kirby, N., Robinson, T., Nigam, P., Banat, I., Marchant, R., and Smyth, W. 2001. Microbial decolourisation and degradation of textile dyes. Appl. Microbiol. Biotechnol.56, 81–87.
Meier-Kolthoff, J.P., Auch, A.F., Klenk, H.P., and Göker, M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics14, 60.
Minnikin, D.E., O’Donnell, A.G., Goodfellow, M., Alderson, G., Athalye, M., Schaal, A., and Parlett, J.H. 1984. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J. Microbiol. Methods2, 233–241.
Minnikin, D.E., Patel, P.V., Alshamaony, L., and Goodfellow, M. 1977. Polar lipid composition in the classification of Nocardia and related bacteria. Int. J. Syst. Evol. Microbiol.27, 104–117.
Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., and Yolken, R.H. 1999. Manual of Clinical Microbiology, 7th edn. American Society for Microbiology press, Washington DC, USA.
Na, S.I., Kim, Y.O., Yoon, S.H., Ha, S.M., Baek, I., and Chun, J. 2018. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol.56, 280–285.
Olender, A., Rutyna, P., Niemcewicz, M., Bogut, A., Ciesielka, M., and Teresiński, G. 2020. Draft whole-genome sequence of Brevibacterium casei strain isolated from a bloodstream infection. Braz. J. Microbiol.51, 685–689.
Overbeek, R., Olson, R., Pusch, G., Olsen, G., Davis, J., Disz, T., Edwards, R.A., Gerdes, S., Parrello, B., Shukla, M., et al. 2013. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res.42, D206–D214.
Pascual, C. and Collins, M.D. 1999. Brevibacterium avium sp. nov., isolated from poultry. Int. J. Syst. Bacteriol.49, 1527–1530.
Rattray, F.P. and Fox, P.F. 1999. Aspects of enzymology and biochemical properties of Brevibacterium linens relevant to cheese ripening: a review. J. Dairy Sci.82, 891–909.
Roux, V. and Raoult, D. 2009. Brevibacterium massiliense sp. nov., isolated from a human ankle discharge. Int. J. Syst. Evol. Microbiol.59, 1960–1964.
Saitou, N.M. and Nei, M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol.4, 406–425.
Sasser, M. 1990. Identification of bacteria through fatty acid analysis. In Klement, Z., Rudolph, K., and Sands, D.C. (eds.), Methods in Phytobacteriology, pp. 199–204. Akadémiai Kaidó, Budapest, Hungary.
Simoes, F., Vale, P., Stephenson, T., and Soares, A. 2018. Understanding the growth of the bio-struvite production Brevibacterium antiquum in sludge liquors. Environ. Technol.39, 2278–2287.
Solano, F., Lucas-Elío, P., López-Serrano, D., Fernández, E., and Sanchez-Amat, A. 2001. Dimethoxyphenol oxidase activity of different microbial blue multicopper proteins. FEMS Microbiol. Lett.204, 175–181.
Staneck, J.L. and Roberts, G.D. 1974. Simplified approach to identification of aerobic actinomycetes by Thin-Layer Chromatography. Appl. Microbiol.28, 226–231.
Wang, W., Zhang, Z., Ni, H., Yang, X., Li, Q., and Li, L. 2012. Decolorization of industrial synthetic dyes using engineered Pseudomonas putida cells with surface-immobilized bacterial laccase. Microb. Cell Fact.11, 75.
Yin, X., Yang, Y., Wang, S., and Zhang, G. 2015. Virgibacillus oceani sp. nov. isolated from ocean sediment. Int. J. Syst. Evol. Microbiol.65, 159–164.
Yoon, S.H., Ha, S.M., Lim, J., Kwon, S., and Chun, J. 2017. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie van Leeuwenhoek110, 1281–1286.
Acknowledgements
This work was supported by grants from the Scientific Research Foundation of Third Institute of Oceanography, MNR (No. 2019011) and China Ocean Mineral Resources R&D Association (COMRA) Program (No. DY135-B2-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there are no conflicts of interest.
Additional information
Supplemental material for this article may be found at http://www.springerlink.com/content/120956.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pei, S., Niu, S., **e, F. et al. Brevibacterium limosum sp. nov., Brevibacterium pigmenatum sp. nov., and Brevibacterium atlanticum sp. nov., three novel dye decolorizing actinobacteria isolated from ocean sediments. J Microbiol. 59, 898–910 (2021). https://doi.org/10.1007/s12275-021-1235-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-021-1235-0