Abstract
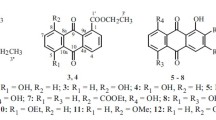
Two new quinones, 1-hydroxy-5-pentyl-anthraquinone (1) and 4-(5-hydroxy-1,4-dioxo-1,4-dihydro-naphthalen-2-ylamino)-butyric acid methyl ester (2), together with two known quinones, 5-hydroxy-2-(2-hydroxy-ethylamino)-(1,4) naphthoquinone (3) and juglone (4) were isolated from the roots of Juglans mandshurica (Juglandaceae). Their structures were elucidated on the basis of spectral data. Compound 3 was isolated from the Juglans genus for the first time. Compounds 1–4 exhibited significant cytotoxicity towards cultured MDA-MB231, HepG2 and SNU638 cells with IC50 values ranging from 4.46 to 88.47 μM.


Similar content being viewed by others
References
Bhasin D, Chettiar SN, Etter JP, Mok M, Li PK (2013) Anticancer activity and SAR studies of substituted 1,4-naphthoquinones. Bioorgan Med Chem 21:4662–4669
Castillo G, Ellames GJ, Osborne AG, Sammes PG (1978) Use of long-range carbon-13-proton coupling constants for structural assignments of juglone derivatives. J Chem Res-S 2:45
Ju SM, Song HY, Lee SJ, Seo WY, Sin DH, Goh AR, Kang YH, Kang IJ, Won MH, Yi JS, Kwon DJ, Bae YS, Choi SY, Park J (2009) Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta-O-galloyl-β-d-glucose via blockade of NF-kappaB and STAT1 activation in the HaCaT cells. Biochem Biophys Res Commun 387:115–120
Kim SH, Lee KS, Son JK, Je GH, Lee JS, Lee CH, Cheong CJ (1998) Cytotoxic compounds from the roots of Juglans mandshurica. J Nat Prod 61:643–645
Lee SW, Lee KS, Son JK (2000) New naphthalenyl glycosides from the roots of Juglans mandshurica. Planta Med 66:184–186
Lee KS, Li G, Kim SH, Lee CS, Woo MH, Lee SH, Jhang YD, Son JK (2002) Cytotoxic diarylheptanoids from the roots of Juglans mandshurica. J Nat Prod 65:1707–1708
Li G, Lee SY, Lee KS, Lee SW, Kim SH, Lee SH, Lee CS, Woo MH, Son JK (2003a) DNA topoisomerases I and II inhibitory activity of constituents isolated from Juglans mandshurica. Arch Pharm Res 26:466–470
Li G, Xu ML, Choi HG, Lee SH, Jahng YD, Lee CS, Moon DC, Woo MH, Son JK (2003b) Four new diarylheptanoids from the roots of Juglans mandshurica. Chem Pharm Bull 51:262–264
Li G, Seo CS, Lee SH, Jahng YD, Chang HW, Lee CS, Woo MH, Son JK (2004) Diarylheptanoids from the roots of Juglans mandshurica. Bull Korean Chem Soc 25:397–399
Li G, Cui JM, Kwon YJ, Seo CS, Lee CS, Woo MH, Lee ES, Jahng YD, Chang HW, Lee SH, Son JK (2005) Two new diarylheptanoids from Juglans mandshurica. Bull Korean Chem Soc 26:1878–1880
Li ZB, Wang JY, Jiang B, Zhang XL, An LJ, Bao YM (2007) Benzobijuglone, a novel cytotoxic compound from Juglans mandshurica, induced apoptosis in HeLa cervical cancer cells. Phytomedicine 14:846–852
Li ZB, Wang JY, Yang J, Zhang XL, An LJ, Bao YM (2009) Apoptosis of BGC823 cell line induced by p-hydroxymethoxybenzobijuglone, a novel compound from Juglans mandshurica. Phytother Res 23:551–557
Lin H, Zhang YW, Bao YL, Wu Y, Sun LG, Yu CL, Huang YX, Wang EB, Li YX (2013) Secondary metabolites from the stem bark of Juglans mandshurica. Biochem Syst Ecol 51:184–188
Min BS, Nakamura N, Miyashiro H, Kim YH, Hattori M (2000) Inhibition of human immunodeficiency virus type 1 reverse transcriptase and ribonuclease H activities by constituents of Juglans mandshurica. Chem Pharm Bull 48:194–200
Min BS, Lee HK, Lee SM, Kim YH, Bae KH, Otake T, Nakamura N, Hattori M (2002) Anti-human immunodeficiency virus-type 1 activity of constituents from Juglans mandshurica. Arch Pharm Res 25:441–445
Min BS, Lee SY, Kim JH, Lee JK, Kim TJ, Kim DH, Kim YH, Joung H, Lee HK, Nakamura N, Miyashiro H, Hattori M (2003) Anti-complement activity of constituents from the stem-bark of Juglans mandshurica. Biol Pharm Bull 26:1042–1044
Ngoc TM, Hung TM, Thuong PT, Kim JC, Choi JS, Bae K, Hattori M, Choi CS, Lee JS, Min BS (2008) Antioxidative activities of galloyl glucopyranosides from the stem-bark of Juglans mandshurica. Biosci Biotechnol Biochem 72:2158–2163
Park G, Oh MS (2014) Inhibitory effects of Juglans mandshurica leaf on allergic dermatitis-like skin lesions-induced by 2,4-dinitrochlorobenzene in mice. Exp Toxicol Pathol 66:97–101
Park GH, Jang DS, Oh MS (2012) Juglans mandshurica leaf extract protects skin fibroblasts from damage by regulating the oxidative defense system. Biochem Biophys Res Commun 421:343–348
Rebelo SLH, Simões MMQ, Neves MGPMS, Silva AMS, Tagliatesta P, Cavaleiro JAS (2005) Oxidation of bicyclic arenes with hydrogen peroxide catalysed by Mn(III) porphyrins. J Mol Catal A-Chem 232:135–142
Son JK (1995) Isolation and structure determination of a new tetralone glucoside from the roots of Juglans mandshurica. Arch Pharm Res 18:203–205
Tian H, Ip L, Luo H, Chang DC, Luo KQ (2007) A high throughput drug screen based on fluorescence resonance energy transfer (FRET) for anticancer activity of compounds from herbal medicine. Br J Pharmacol 150:321–334
Yao Y, Zhang YW, Sun LG, Liu B, Bao YL, Lin H, Zhang Y, Zheng LH, Sun Y, Yu CL, Wu Y, Wang GN, Li YX (2012) Juglanthraquinone C, a novel natural compound derived from Juglans mandshurica Maxim, induces S phase arrest and apoptosis in HepG2 cells. Apoptosis 17:832–841
Yao D, ** M, Zhang C, Luo J, Li R, Zheng M, Cui J, Li G (2014) A new phenolic glycoside from Juglans mandshurica. Nat Prod Res 28:998–1002
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grant numbers 81160386, 81260474, and 30911140276. This work was also partially supported by Jilin Provincial Science & Technology Department under Grant number 20140101037JC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
**, M., Sun, J., Li, R. et al. Two new quinones from the roots of Juglans mandshurica . Arch. Pharm. Res. 39, 1237–1241 (2016). https://doi.org/10.1007/s12272-016-0781-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0781-1