Abstract
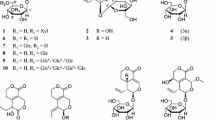
Radix Astragali (Astragalus membranaceus) is an important traditional Chinese medicine that is widely used as a tonic to enhance the body’s natural defense mechanisms. In this phytochemical study, 12 flavonoids, isoliquiritigenin (1), liquiritigenin (2), calycosin (3), calycosin 7-O-β-d-glucoside (4), formononetin (5), formononetin 7-O-β-d-glucoside (6), daidzein (7), daidzein 7-O-β-d-glucoside (8), methylnissolin (9), methylnissolin 3-O-β-d-glucoside (10), isomucronulatol (11), and isomucronulatol 7-O-β-d-glucoside (12), were isolated from the roots of A. membranaceus. Their structures were elucidated by comparing spectroscopic data with reported values. The effects of the isolated compounds on lipopolysaccharide (LPS)-stimulated bone marrow-derived dendritic cells were investigated. Compounds 1 and 2 exhibited significant inhibitory effects on LPS-induced IL-6 and IL-12 p40 production, with IC50 values ranging from 2.7 to 6.1 μM. Compound 1 also showed a moderate inhibitory effect on LPS-stimulated production of TNF-α with an IC50 value of 20.1 μM. Further studies of the potential anti-inflammatory effects and benefits of flavonoids from A. membranaceus are warranted.



Similar content being viewed by others
References
Anas, S., O. Yoshiteru, and H. Hiroshi. 1991. Isoflavans and a pterocarpan from Astragalus mongholicus. Phytochemistry 30: 2777–2780.
Beutler, B., and A. Cerami. 1986. Cachectin and tumor necrosis factor as two sides of the same biological coin. Nature 320: 584–588.
Chen, H.J., C.P. Chung, W. Chiang, and Y.L. Lin. 2011. Anti-inflammatory effects and chemical study of a flavonoid-enriched fraction from adlay bran. Food Chemistry 126: 1741–1748.
Choi, C.W., Y.H. Choi, M.R. Cha, D.S. Yoo, Y.S. Kim, G.H. Yon, K.S. Hong, Y.H. Kim, and S.Y. Ryu. 2010. Yeast α-glucosidase inhibition by isoflavones from plants of Leguminosae as an in vitro alternative to acarbose. Journal of Agriculture and Food Chemistry 58: 9988–9993.
Choi, S.I., T.R. Heo, B.H. Min, J.H. Cui, B.H. Choi, and S.R. Park. 2007. Alleviation of osteoarthritis by calycosin-7-O-β-d-glucopyranoside (CG) isolated from Astragali Radix (AR) in rabbit osteoarthritis (OA) model. Osteoarthritis Cartilage 15: 1086–1092.
Cooper, A.M., and S.A. Khader. 2007. IL-12 p40: An inherently agonistic cytokine. Trends in Immunology 28: 33–38.
Cushnie, T.P.T., and A.J. Lamb. 2005. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents 26: 343–356.
Cushnie, T.P.T., and A.J. Lamb. 2011. Recent advances in understanding the antibacterial properties of flavonoids. International Journal of Antimicrobial Agents 38: 99–107.
de Sousa, R.R., K.C. Queiroz, A.C. Souza, S.A. Gurgueira, A.C. Augusto, M.A. Miranda, M.P. Peppelenbosch, C.V. Ferreira, and H. Aoyama. 2007. Phosphoprotein levels, MAPK activities and NF-κB expression are affected by fisetin. Journal of Enzyme Inhibition and Medicinal Chemistry 22: 439–444.
Gately, M.K., L.M. Renzetti, J. Magram, A.S. Stern, L. Adorini, U. Gubler, and D.H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: Role in normal and pathologic immune responses. Annual Review of Immunology 16: 495–521.
Jiao, Y., J. Wen, and X. Yu. 1999. Influence of flavonoid of Astragalus membranaceus’s stem and leaves on the function of cell mediated immunity in mice. Zhongguo Zhong ** Yi Jie He Za Zhi 19: 356–358.
**, S.E., Y.K. Son, B.S. Min, H.A. Jung, and J.S. Choi. 2012. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Archives of Pharmacal Research 35: 823–837.
Kiem, P.V., C.V. Minh, N.X. Nhiem, N.X. Cuong, B.H. Tai, T.H. Quang, H.L.T. Anh, P.H. Yen, N.K. Ban, S.H. Kim, M. **n, J.Y. Cha, Y.M. Lee, and Y.H. Kim. 2012. Inhibitory effect on TNF-α-induced IL-8 secretion in HT-29 cell line by glyceroglycolipids from the leaves of Ficus microcarpa. Archives of Pharmacal Research 35: 2135–2142.
Kim, Y.W., R.J. Zhao, S.J. Park, J.R. Lee, I.J. Cho, C.H. Yang, S.G. Kim, and S.C. Kim. 2008. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappa B-dependent iNOS and proinflammatory cytokines production. British Journal of Pharmacology 154: 165–173.
Kulesh, N.I., N.A. Vasilevskaya, M.V. Veselova, V.A. Denisenko, and S.A. Fedoreev. 2008. Minor polyphenols from Maackia amurensis wood. Chemistry of Natural Compounds 44: 712–714.
Lai, P.K., J.Y. Chan, L. Cheng, C.P. Lau, S.Q. Han, P.C. Leung, K.P. Fung, and C.B. Lau. 2012. Isolation of anti-inflammatory fractions and compounds from the root of Astragalus membranaceus. Phytotherapy Research. doi:10.1002/ptr.4759.
Lau, B.H.S., P. Ong, and J. Tosk. 1989. Macrophage chemiluminescence modulated by Chinese medicinal herbs Astragalus membranaceus and Ligustrum luidum. Phytotherapy Research 3: 148–153.
Lee, J.C., J.T. Laydon, P.C. McDonnell, T.F. Gallagher, S. Kumar, D. Green, D. McNulty, M.J. Blumenthal, J.R. Heys, and S.W. Landvatter. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372: 739–746.
Lee, Y.S., O.K. Han, C.W. Park, C.H. Yang, T.W. Jeon, W.K. Yo, S.H. Kim, and H.J. Kim. 2005. Pro-inflammatory cytokine gene expression and nitric oxide regulation of aqueous extracted Astragali radix in RAW 264.7 macrophage cells. Journal of Ethnopharmacology 100: 289–294.
Li, W., Y. Asada, and T. Yoshikawa. 2000. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry 55: 447–456.
Ma, X.Q., Q. Shi, J.A. Duan, D.Y. Zhu, T.T.X. Dong, and K.W.K. Tsim. 2002. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. Journal of Agriculture and Food Chemistry 50: 4861–4866.
Mannon, P.J., I.J. Fuss, L. Mayer, C.O. Elson, W.J. Sandborn, D. Present, B. Dolin, N. Goodman, C. Groden, R.L. Hornung, M. Quezado, M.F. Neurath, J. Salfeld, G.M. Veldman, U. Schwertschlag, W. Strober, and Anti–IL-12 Crohn’s Disease Study Group. 2004. Anti–interleukin-12 antibody for active Crohn’s disease. New England Journal of Medicine 351: 2069–2079.
Mersereau, J.E., N. Levy, R.E. Staub, S. Baggett, T. Zogovic, S. Chow, W.A. Ricke, M. Tagliaferri, I. Cohen, L.F. Bjeldanes, and D.C. Leitman. 2008. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Molecular and Cellular Endocrinology 283: 49–57.
Papanicolaou, D.A., R.L. Wilder, S.C. Manolagas, and G.P. Chrousos. 1998. The pathophysiologic roles of interleukin-6 in human disease. Annals of Internal Medicine 128: 127–137.
Purmová, J., and L. Opletal. 1995. Phytotherapeutic aspects of diseases of the cardiovascular system. 5. Saponins and possibilities of their use in prevention and therapy. Ceska A Slovenska Farmacie 44: 246–251.
Salem, M.M., and K.A. Werbovetz. 2006. Isoflavonoids and other compounds from Psorothamnus arborescens with antiprotozoal activities. Journal of Natural Products 69: 43–49.
Saklatvala, J. 2004. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Current Opinion in Pharmacology 4: 372–377.
Schuier, M., H. Sies, B. Illek, and H. Fischer. 2005. Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia. Journal of Nutrition 135: 2320–2325.
Sugamoto, K., Y. Matsusita, K. Matsui, C. Kurogi, and T. Matsui. 2011. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 67: 5346–5359.
Tung, N.H., T.H. Quang, J.H. Son, J.E. Koo, H.J. Hong, Y.S. Koh, G.Y. Song, and Y.H. Kim. 2011. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Archives of Pharmacal Research 34: 681–685.
Wang, D.C. 1989. Influence of Astragalus membranaceus (AM) polysaccharide FB on immunologic function of human periphery blood lymphocyte. Zhongguo Zhong Yao Za Zhi 11: 180–183.
Weinstein, D.L., B.L. O’Neill, and E.S. Metcalf. 1997. Salmonella typhi stimulation of human intestinal epithelial cells induces secretion of epithelial cell-derived interleukin-6. Infection and Immunity 65: 395–404.
Zhang, L.J., H.K. Liu, P.C. Hsiao, L.M. Kuo, I.J. Lee, T.S. Wu, W.F. Chiou, and Y.H. Kuo. 2011. New isoflavonoid glycosides and related constituents from Astragali Radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. Journal of Agriculture and Food Chemistry 59: 1131–1137.
Zhao, K.S., C. Mancini, and G. Doria. 1990. Enhancement of the immune response in mice by Astragalus membranaceus extracts. Immunopharmacology 20: 225–233.
Zhao, X.Z. 1992. Effects of Astragalus membranaceus and Tripterygium hypoglancum on natural killer cell activity of peripheral blood mononuclear in systemic lupus erythematosus. Zhongguo Zhong ** Yi Jie He Za Zhi 12: 671–699.
Acknowledgments
This study was supported by the Priority Research Center Program (2009-0093815) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, and the Ministry of Knowledge Economy (MKE), Korea Institute for Advancement of Technology (KIAT) and Honam Leading Industry Office through the Leading Industry Development for Economic Region, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Sun, Y.N., Yan, X.T. et al. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharm. Res. 37, 186–192 (2014). https://doi.org/10.1007/s12272-013-0174-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0174-7