Abstract
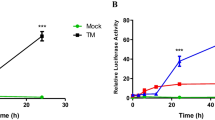
Accumulation of mis- and unfolded proteins during viral replication can cause stress in the endoplasmic reticulum (ER) and trigger the unfolded protein response (UPR). If unchecked, this process may induce cellular changes detrimental to viral replication. In the report, we investigated the impact of HSV-1 on the UPR during lytic replication. We found that HSV-1 effectively disarms the UPR in early stages of viral infection. Only ATF6 activation was detected during early infection, but with no upregulation of target chaperone proteins. Activity of the eIF2α/ATF4 signaling arm increased at the final stage of HSV-1 replication, which may indicate completion of virion assembly and egress, thus releasing suppression of the UPR. We also found that the promoter of viral ICP0 was responsive to ER stress, an apparent mimicry of cellular UPR genes. These results suggest that HSV-1 may use ICP0 as a sensor to modulate the cellular stress response.








Similar content being viewed by others
References
Audas TE, Li Y, Liang G, Lu R (2008) A novel protein, Luman/CREB3 recruitment factor, inhibits Luman activation of the unfolded protein response. Mol Cell Biol 28(12):3952–3966
Bernales S, Papa FR, Walter P (2006) Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol 22:487–508
Buchkovich NJ, Maguire TG, Yu Y, Paton AW, Paton JC, Alwine JC (2008) Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly. J Virol 82(1):31–39
Cai WZ, Schaffer PA (1989) Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol 63(11):4579–4589
Cai W, Schaffer PA (1992) Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol 66(5):2904–2915
Cao G, Ni X, Jiang M, Ma Y, Cheng H, Guo L, Ji C, Gu S, **e Y, Mao Y (2002) Molecular cloning and characterization of a novel human cAMP response element-binding (CREB) gene (CREB4). J Hum Genet 47(7):373–376
Cassady KA, Gross M, Roizman B (1998) The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J Virol 72(11):8620–8626
Chao CC, Yam WC, Chen LK, Lin-Chao S (1992) Cloning of a functional Burkitt's lymphoma polypeptide-binding protein/78 kDa glucose-regulated protein (BiP/GRP78) gene promoter by the polymerase chain reaction, and its interaction with inducible cellular factors. Biochem J 286(Pt 2):555–559
Chin K-T, Zhou H-J, Wong C-M, Lee JM-F, Chan C-P, Qiang B-Q, Yuan J-G, Ng IO-l, ** D-Y (2005) The liver-enriched transcription factor CREB-H is a growth suppressor protein underexpressed in hepatocellular carcinoma. Nucl Acids Res 33(6):1859–1873
Elgadi MM, Hayes CE, Smiley JR (1999) The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J Virol 73(9):7153–7164
Everett RD (1988) Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J Mol Biol 202(1):87–96
Everett RD (2000) ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22(8):761–770
Everett RD, Boutell C, Orr A (2004) Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J Virol 78(4):1763–1774
Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T (1992) Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol 66(1):341–348
Freiman RN, Herr W (1997) Viral mimicry: common mode of association with HCF by VP16 and the cellular protein LZIP. Genes Dev 11(23):3122–3127
Freire E, Oddo C, Frappier L, de Prat-Gay G (2008) Kinetically driven refolding of the hyperstable EBNA1 origin DNA-binding dimeric beta-barrel domain into amyloid-like spherical oligomers. Proteins 70(2):450–461
Halford WP, Schaffer PA (2001) ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J Virol 75(7):3240–3249
Halford WP, Kemp CD, Isler JA, Davido DJ, Schaffer PA (2001) ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J Virol 75(13):6143–6153
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6(5):1099–1108
He B, Chou J, Liebermann DA, Hoffman B, Roizman B (1996) The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the gamma(1)34.5 gene of herpes simplex virus to preclude the premature shutoff of total protein synthesis in infected human cells. J Virol 70(1):84–90
Honma Y, K-y K, Mori T, Tanno Y, Tojo M, Kiyosawa H, Takeda J, Nikaido T, Tsukamoto T, Yokoya S, Wanaka A (1999) Identification of a novel gene, OASIS, which encodes for a putative CREB/ATF family transcription factor in the long-term cultured astrocytes and gliotic tissue. Mol Brain Res 69(1):93–103
Isler JA, Skalet AH, Alwine JC (2005) Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol 79(11):6890–6899
Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC (2004) Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol 24(3):1365–1377
Jordan R, Wang L, Graczyk TM, Block TM, Romano PR (2002) Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J Virol 76(19):9588–9599
Laurent AM, Madjar JJ, Greco A (1998) Translational control of viral and host protein synthesis during the course of herpes simplex virus type 1 infection: evidence that initiation of translation is the limiting step. J Gen Virol 79(Pt 11):2765–2775
Lee DY, Sugden B (2008) The LMP1 oncogene of EBV activates PERK and the unfolded protein response to drive its own synthesis. Blood 111(4):2280–2289
Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PA (1989) Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol 63(2):759–768
Li B, Gao B, Ye L, Han X, Wang W, Kong L, Fang X, Zeng Y, Zheng H, Li S, Wu Z, Ye L (2007) Hepatitis B virus X protein (HBx) activates ATF6 and IRE1-XBP1 pathways of unfolded protein response. Virus Res 124(1–2):44–49
Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn AC, Kokame K, Lu R (2006) Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol 26(21):7999–8010
Lu R, Yang P, O'Hare P, Misra V (1997) Luman, a new member of the CREB/ATF family, binds to herpes simplex virus VP16-associated host cellular factor. Mol Cell Biol 17(9):5117–5126
Lu PD, Harding HP, Ron D (2004) Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol 167(1):27–33
Malhotra JD, Kaufman RJ (2007) The endoplasmic reticulum and the unfolded protein response. Sem Cell Mol Biol 18(6):716–731
Mori K (2009) Signaling pathways in the unfolded protein response: development from yeast to mammals. J Biochem. doi:10.1093/jb/mvp166
Mulvey M, Poppers J, Sternberg D, Mohr I (2003) Regulation of eIF2alpha phosphorylation by different functions that act during discrete phases in the herpes simplex virus type 1 life cycle. J Virol 77(20):10917–10928
Mulvey M, Arias C, Mohr I (2006) Resistance of mRNA translation to acute endoplasmic reticulum stress-inducing agents in herpes simplex virus type 1-infected cells requires multiple virus-encoded functions. J Virol 80(15):7354–7363
Mulvey M, Arias C, Mohr I (2007) Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1-infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J Virol 81(7):3377–3390
Nagamori I, Yabuta N, Fujii T, Tanaka H, Yomogida K, Nishimune Y, Nojima H (2005) Tisp40, a spermatid specific bZip transcription factor, functions by binding to the unfolded protein response element via the Rip pathway. Genes Cells 10(6):575–594
Omori Y, J-i I, Watanabe M, Komatsu T, Suzuki Y, Kataoka K, Watanabe S, Tanigami A, Sugano S (2001) CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nucl Acids Res 29(10):2154–2162
Pahl HL, Sester M, Burgert HG, Baeuerle PA (1996) Activation of transcription factor NF-kappaB by the adenovirus E3/19K protein requires its ER retention. J Cell Biol 132(4):511–522
Preston CM (2007) Reactivation of expression from quiescent herpes simplex virus type 1 genomes in the absence of immediate-early protein ICP0. J Virol 81(21):11781–11789
Qi H, Fillion C, Labrie Y, Grenier J, Fournier A, Berger L, El-Alfy M, Labrie C (2002) AIbZIP, a novel bZIP gene located on chromosome 1q21.3 that is highly expressed in prostate tumors and of which the expression is up-regulated by androgens in LNCaP human prostate cancer cells. Cancer Res 62(3):721–733
Raggo C, Rapin N, Stirling J, Gobeil P, Smith-Windsor E, O'Hare P, Misra V (2002) Luman, the cellular counterpart of herpes simplex virus VP16, is processed by regulated intramembrane proteolysis. Mol Cell Biol 22(16):5639–5649
Schroder M (2008) Endoplasmic reticulum stress responses. Cell Mol Life Sci 65(6):862–894
Sir D, Chen WL, Choi J, Wakita T, Yen TS, Ou JH (2008a) Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology 48(4):1054–1061
Sir D, Liang C, Chen WL, Jung JU, Ou JH (2008b) Perturbation of autophagic pathway by hepatitis C virus. Autophagy 4(6):830–831
Stelzer G, Don J (2002) Atce1: a novel mouse cyclic adenosine 3',5'-monophosphate-responsive element-binding protein-like gene exclusively expressed in postmeiotic spermatids. Endocrinology 143(5):1578–1588
Stirling J, O'Hare P (2006) CREB4, a transmembrane bZip transcription factor and potential new substrate for regulation and cleavage by S1P. Mol Biol Cell 17(1):413–426
Storlazzi CT, Mertens F, Nascimento A, Isaksson M, Wejde J, Brosjo O, Mandahl N, Panagopoulos I (2003) Fusion of the FUS and BBF2H7 genes in low grade fibromyxoid sarcoma. Hum Mol Genet 12(18):2349–2358
Stow ND, Stow EC (1986) Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol 67(Pt 12):2571–2585
Su HL, Liao CL, Lin YL (2002) Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J Virol 76(9):4162–4171
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13(3):184–190
Talloczy Z, Virgin HWT, Levine B (2006) PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy 2(1):24–29
Tardif KD, Mori K, Siddiqui A (2002) Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J Virol 76(15):7453–7459
Thompson RL, Sawtell NM (2006) Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J Virol 80(22):10919–10930
Tirasophon W, Welihinda AA, Kaufman RJ (1998) A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev 12(12):1812–1824
Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A 101(31):11269–11274
Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W (2003) Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev 17(7):896–911
Xuan B, Qian Z, Torigoi E, Yu D (2009) Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J Virol 83(8):3463–3474
Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K (2004) Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem (Tokyo) 136(3):343–350
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13(3):365–376
Yao F, Schaffer PA (1994) Physical interaction between the herpes simplex virus type 1 immediate-early regulatory proteins ICP0 and ICP4. J Virol 68(12):8158–8168
Yao F, Schaffer PA (1995) An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol 69(10):6249–6258
Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20(18):6755–6767
Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K (2001) Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol Cell Biol 21(4):1239–1248
Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K (2003) A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4(2):265–271
Acknowledgement
We would like to thank Randal Kaufman for the IRE1−/− cells, Karen Mossman for the HSV-1 KOS and the 7134 strains, and Ron Prywesfor the pGL3-5 × UPRE-luciferase plasmid. This work was supported by funds from the Canadian Institutes for Health Research and Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Heather F. Burnett and Timothy E. Audas contributed equally to this work.
Rights and permissions
About this article
Cite this article
Burnett, H.F., Audas, T.E., Liang, G. et al. Herpes simplex virus-1 disarms the unfolded protein response in the early stages of infection. Cell Stress and Chaperones 17, 473–483 (2012). https://doi.org/10.1007/s12192-012-0324-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-012-0324-8