Abstract
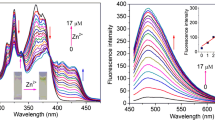
A novel Schiff base derivative (Z)-3-(4-(hexyloxy)phenyl)-2-(4-((E)-2-hydroxybenzylidene amino)phenyl)acrylonitrile (L) was designed, synthesized and characterized. L was used as a Zn2+ selective, turn-on, fluorescent chemosensor with a detection limit of 0.1 μM in DMF. 1:1 stoichiometric complex formation of L wih Zn2+ was confirmed through fluorescent titration experiments and Job’s plot. The enhancement of fluorescence intensity of L with addition of Zn2+ is the consequence of the inhibited isomerization of the C=N bond, namely chelation-enhanced fluorescence (CHEF) effect.

α-Cyanostilbene-modified Schiff L was designed as a highly selective and sensitive zinc chemosensor. The complexation of L with Zn2+ enhanced the fluorescence intensity. This chemosensor could distinguish clearly Zn2+ from Cd2+, a frequently encountered trouble in the design for the Zn2+ fluorescent probes.









Similar content being viewed by others
References
Carter K P, You ng A M and Palmer A E 2014 Chem. Rev. doi: 10.1021/cr400546e
Liu T and Liu S Y 2011 Anal. Chem. 83 2775
Carter R E, Weiss J H and Shuttleworth C W 2010 Neuroreport 21 1060
Maret W, Jacob C, Vallee B L and Fisher E H 1999 Proc. Natl. Acad. Sci. 96 1936
An J M, Yan M H, Yang Z Y, Li T R and Zhou Q X 2013 Dyes Pigments 99 1
Kong L, Chen Y, Ye W B and Zhao L, Song B 2013 Sens. Actuators B 177 218
Zhang Y, Guo X F, Zheng L, Jia L H and Qian X H 2013 J. Photochem. Photobiol. A 257 20
Liu X J, Zhang N, Zhou J, Chang T J, Fang C L and Shangguan D H 2013 Analyst 138 901
Safin D A, Babashkina M G and Garcia Y 2013 Dalton Trans. 42 1969
Goswami S, Das A K, Aich K, Manna A, Maity S, Khanra K and Bhattacharyya N 2013 Analyst 138 4593
Jiao S Y, Peng L L, Li K, **e Y M, Ao M Z, Wang X and Yu X Q 2013 Analyst 138 5762
Du J, Huang Z, Yu X Q and Pu L 2013 Chem. Commun. 49 5399
Mikata Y, Sato Y, Takeuchi S, Kuroda Y, Konno H and Iwatsuki S 2013 Dalton Trans. 42 9688
Wang L, Qin W W and Liu W S 2010 Inorg. Chem. Commun. 13 1122
Helal A and Kim H S 2013 Spectrochim. Acta 105 273
Kumar M, Kumar N and Bhalla V 2013 Chem. Commun. 49 877
Ingale S A and Seela F 2012 J. Org. Chem. 77 9352
Pearce D A, Jotterand N, Carrico I S and Imperiali B 2001 J. Am. Chem. Soc. 123 5160
Zhang Y, Guo X F, Si W X, Jia L H and Qian X H 2008 Org. Lett. 10 473
Ding Y B, **e Y S, Li X, Hill J P, Zhang W B and Zhu W H 2011 Chem. Commun. 47 5431
Thirupathi P and Lee K H 2013 Bioorg. Med. Chem. Lett. 23 6811
Tong A J, Dong H and Li L D 2002 Anal. Chim. Acta 46 31
Xu Z C. Qian X H, Cui J N and Zhang R 2006 Tetrahedron 62 10117
Nolan E M and Lippard S J 2004 Inorg. Chem. 43 8310
Aoki S, Kagata D, Shiro M, Takeda K and Kimura E 2004 J. Am. Chem. Soc. 126 13377
Wang P F, Hong Z R, **e Z Y, Tong S, Wong O, Lee C S, Wong N, Hung L and Lee S 2003 Chem. Commun. 1664
Yu T Z, Zhang K, Zhao Y L, Yang C H, Zhang H, Qian L, Fan D W, Dong W K, Chen L L and Qiu Y Q 2008 Inorg. Chim. Acta 361 233
**e Y Z, Shan G G, Li P, Zhou Z Y and Su Z M 2013 Dyes Pigments 96 467
Navarro M, Castro W, Martíznez A and Delgado R A S 2011 J. Inorg. Biochem. 105 276
Mishra A P, Mishra R K and Pandey M D 2011 Russ. J. Inorg. Chem. 56 1757
Aziz A A A, Badr I H A and El-Syaed I S A 2012 Spectrochim. Acta A 97 388
Wang S C, Men G W, Zhao L Y, Hou Q F and Jiang S M 2010 Sens. Actuators B 145 826
Zang L B, Shang H G, Wei D Y and Jiang S M 2013 Sens. Actuators B 185 389
Hiseh W H, Wan C F, Liao D J and Wu A T 2012 Tetrahedron Lett. 53 5848
Ding C X, He C H and **e Y S 2013 Chinese Chem. Lett. 24 463
Liu S B, Bi C F, Fan Y H, Zhao Y, Zhang P F, Luo Q D and Zhang D M 2011 Inorg. Chem. Commun. 14 1297
Hosseini M, Vaezi Z, Ganjali M R, Faridbod F, Abkenar S D, Alizadeh K and Niasari M S 2010 Spectrochim. Acta A 5 978
Udhayakumari D, Saravanamoorthy S, Ashok M and Velmathi S 2011 Tetrahedron Lett. 52 4631
Paul B K, Kar S and Guchhait N 2011 J. Photochem. Photobio. A 220 153
Li H Y, Gao S and ** Z 2009 Inorg. Chem. Commun. 12 300
An B K, Kwon S K, Jung S D and Park S Y 2002 J. Am. Chem. Soc. 124 14410
Chung J W, You Y, Huh H S, An B K, Yoon S J, Kim S H, Lee S W and Park S Y 2009 J. Am. Chem. Soc. 131 8163
Dou C D, Han L, Zhao S S, Zhang H Y and Wang Y 2011 J. Phys. Chem. Lett. 2 666
Zhu L L and Zhao Y L 2013 J. Mater. Chem. C 1 1059
Lim C K, Kim S, Kwon I C, Ahn C H and Park S Y 2009 Chem. Mater. 21 5819
Jia W B, Yang P, Li J J, Yin Z M, Kong L, Lu H B, Ge Z S, Wu Y Z, Hao X P and Yang J X 2014 Poly. Chem. 5 2282
Jia W B, Wang H W, Yang L M, Lu H B, Kong L, Tian Y P, Tao X T and Yang J X 2013 J. Mater. Chem. C 1 7092
Gou C, Qin S H, Wu H Q, Wang Y, Luo J and Liu X Y 2011 Inorg. Chem. Commun. 14 1622
Kim B, Yeom H R, Choi W Y, Kim J Y and Yang C 2012 Tetrahedron 68 6696
Lu H B, Qiu L Z, Zhang G Y, Ding A X, Xu W B, Zhang G B, Wang X H, Kong L, Tian Y P and Yang J X 2014 J. Mater. Chem. C 2 1386
Frisch M J G W T, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G J, Sonnenberg L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta Jr J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S, Tomasi S J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas O, Foresman J B, Ortiz J V and Cioslowski J, Fox D J Gaussian 09, Revision B. 01. Gaussian, Inc., Wallingford CT. 2009
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21101001 and 61107014), the Natural Science Foundation of Anhui Province (1208085MB21), the 211 Project of Anhui University, and the Team for Scientific Innovation Foundation of Anhui Province (2006KJ007TD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Spectral data and calculated electron cloud distribution are given (figures S1–S5 and tables S1 and S2) which can be obtained from www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DING, A., TANG, F., WANG, T. et al. A α-cyanostilbene-modified Schiff base as efficient turn-on fluorescent chemosensor for Zn2+ . J Chem Sci 127, 375–382 (2015). https://doi.org/10.1007/s12039-015-0787-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0787-0