Abstract
Apolipoprotein E4 (APOE4) is the major genetic risk factor for sporadic Alzheimer's disease (AD), which is characterized by amyloid β (Aβ) plaques and tau tangles. Though the role of APOE4 in Aβ pathogenesis has been mechanistically defined in rodent models, much less is known regarding the relationship of APOE4 to tau pathogenesis. Recent studies have indicated a possible correlation between APOE isoform-dependent alterations in tau pathology and neurodegeneration. To explore whether neuronal expression of APOE4 triggers tauopathy, here we delivered adeno-associated viruses (AAV) expressing human APOE4 in two different models of tauopathy—rTg4510 and PS19 lines. Intracerebroventricular delivery of AAV-APOE4 in neonatal rTg4510 and PS19 mice resulted in increased APOE4 protein in neurons but did not result in altered phosphorylated tau burden, pretangle tau pathology, or silver-positive tangle pathology. Biochemical analysis of synaptic proteins did not reveal substantial alterations. Our results indicate that over-expression of APOE4 in neurons, using an AAV-mediated approache, is not sufficient to accelerate or otherwise alter the inherent tau pathology that occurs in mice overexpressing mutant human tau.







Similar content being viewed by others
Availability of Data and Materials
All the data supporting the conclusions of this article are included within the article and its supplementary information files.
Abbreviations
- AAV:
-
adeno-associated viruses
- Aβ:
-
amyloid β
- APOE:
-
human apolipoprotein E
- Apoe:
-
mouse apolipoprotein E
- APOE4 TR:
-
APOE E4 allele-targeted replacement
- AD:
-
Alzheimer's disease
- CaMKII:
-
Ca2+/calmodulin-dependent protein kinase II
- CBA:
-
cytomegalovirus enhancer/chicken β-actin
- EGFP:
-
enhanced green fluorescent protein
- GFAP:
-
glial fibrillary acidic protein
- Iba-1:
-
ionized calcium binding adaptor molecule 1
- MAPT:
-
microtubule-associated protein tau
- NFT:
-
neurofibrillary tangle
- PSP:
-
progressive supranuclear palsy
- ptau:
-
phosphorylated tau
- Tg:
-
transgenic
References
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL et al (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261:921–923
Leduc V, Jasmin-Bélanger S, Poirier J. (2010) APOE and cholesterol homeostasis in Alzheimer's disease. Trends Mol Med 16:469–477
Huang Y, Mahley RW (2014) Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis 72:3–12
Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9:106–118
Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J et al (2009) Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A 106:6820–6825
Huang YA, Zhou B, Wernig M, Sudhof TC (2017) ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Abeta secretion. Cell 168:427–441
Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC et al (2011) Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med 3(89):89ra57
Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM et al (2009) Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci 29:6771–6779
DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JA, Aronow BJ et al (2004) ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron 41:193–202
Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F et al (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med 10:719–726
Farfel JM, Yu L, De Jager PL, Schneider JA, Bennett DA (2016) Association of APOE with tau-tangle pathology with and without beta-amyloid. Neurobiol Aging 37:19–25
Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Gifford KA, Bush WS et al (2018) Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol 75:989–998
Zhao N, Liu CC, Van Ingelgom AJ, Linares C, Kurti A, Knight JA, Heckman MG, Diehl NN et al (2018) APOE epsilon2 is associated with increased tau pathology in primary tauopathy. Nat Commun 9:4388
Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S et al (2017) ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549:523–527
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481
Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T et al (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53:337–351
Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N (1999) Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest 103:1579–1586
Chakrabarty P, Rosario A, Cruz P, Siemienski Z, Ceballos-Diaz C, Crosby K, Jansen K, Borchelt DR et al (2013) Capsid serotype and timing of injection determines AAV transduction in the neonatal mice brain. PLoS One 8:e67680
Koller EJ, Gonzalez De La Cruz E, Machula T, Ibanez KR, Lin W-L, Williams T, Riffe CJ, Ryu D et al (2019) Combining P301L and S320F tau variants produces a novel accelerated model of tauopathy. Hum Mol Genet 28:3255–3269. https://doi.org/10.1093/hmg/ddz151
Kuninaka N, Kawaguchi M, Ogawa M, Sato A, Arima K, Murayama S, Saito Y (2015) Simplification of the modified Gallyas method. Neuropathology 35:10–35
Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M et al (2005) Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci 25(46):10637–10647
Piras A, Collin L, Gruninger F, Graff C, Ronnback A (2016) Autophagic and lysosomal defects in human tauopathies: analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol Commun 4:22
Agosta F, Vossel KA, Miller BL, Migliaccio R, Bonasera SJ, Filippi M, Boxer AL, Karydas A et al (2009) Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. Proc Natl Acad Sci U S A 106:018–022
Wisdom NM, Callahan JL, Hawkins KA (2011) The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 32:63–74
Kopeikina KJ, Polydoro M, Tai HC, Yaeger E, Carlson GA, Pitstick R, Hyman BT, Spires-Jones TL (2013) Synaptic alterations in the rTg4510 mouse model of tauopathy. J Comp Neurol 521:1334–1353
Liao F, Li A, **ong M, Bien-Ly N, Jiang H, Zhang Y, Finn MB, Hoyle R et al (2018) Targeting of nonlipidated, aggregated apoE with antibodies inhibits amyloid accumulation. J Clin Invest 128:2144–2155
Kim J, Eltorai AE, Jiang H, Liao F, Verghese PB, Kim J, Stewart FR, Basak JM et al (2012) Anti-apoE immunotherapy inhibits amyloid accumulation in a transgenic mouse model of Abeta amyloidosis. J Exp Med 209:2149–2156
Geifman N, Brinton RD, Kennedy RE, Schneider LS, Butte AJ (2017) Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer's disease. Alz Res Ther 9:10
Morris JC, Roe CM, **ong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA (2010) APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67:122–131
Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Shaw LM, Trojanowski JQ, Aisen PS, Weiner M et al (2010) Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol 67:308–316
Caselli RJ, Walker D, Sue L, Sabbagh M, Beach T (2010) Amyloid load in nondemented brains correlates with APOE e4. Neurosci Lett 473:168–171
Mattsson N, Ossenkoppele R, Smith R, Strandberg O, Ohlsson T, Jogi J, Palmqvist S, Stomrud E et al (2018) Greater tau load and reduced cortical thickness in APOE epsilon4-negative Alzheimer's disease: a cohort study. Alz Res Ther 10:77
Tsuboi Y, Josephs KA, Cookson N, Dickson DW (2003) APOE E4 is a determinant for Alzheimer type pathology in progressive supranuclear palsy. Neurology 6:240–245
Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K (1991) Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res 541:163–166
Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M et al (1994) Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci U S A 91(23):11183–11186
Fleming LM, Weisgraber KH, Strittmatter WJ, Troncoso JC, Johnson GV (1996) Differential binding of apolipoprotein E isoforms to tau and other cytoskeletal proteins. Exp Neurol 138(2):252–260
Liu C, Song X, Nisbet R, Gotz J (2016) Co-immunoprecipitation with tau isoform-specific antibodies reveals distinct protein interactions and highlights a putative role for 2N tau in disease. J Biol Chem 291:8173–8188
Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW (2001) Apolipoprotein E fragments present in Alzheimer's disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci U S A 98:8838–8843
Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Dee FJ, Wyss-Coray T et al (2004) Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci 24:2527–2534
Chakrabarty P, Li A, Ceballos-Diaz C, Eddy JA, Funk CC, Moore B, DiNunno N, Rosario AM et al (2015) IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron 85:519–533
Lesuisse C, Xu G, Anderson J, Wong M, Jankowsky J, Holtz G, Gonzalez V, Wong PC et al (2001) Hyper-expression of human apolipoprotein E4 in astroglia and neurons does not enhance amyloid deposition in transgenic mice. Hum Mol Genet 10:2525–2537
Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y (2006) Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci 26:4985–4994
Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM (2002) Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol Dis 9:305–318
Liao F, Zhang TJ, Jiang H, Lefton KB, Robinson GO, Vassar R, Sullivan PM, Holtzman DM (2015) Murine versus human apolipoprotein E4: differential facilitation of and co-localization in cerebral amyloid angiopathy and amyloid plaques in APP transgenic mouse models. Acta Neuropathol Commun 3:70
Eberle D, Kim RY, Luk FS, de Mochel NS, Gaudreault N, Olivas VR, Kumar N, Posada JM et al (2012) Apolipoprotein E4 domain interaction accelerates diet-induced atherosclerosis in hypomorphic Arg-61 apoe mice. Arterioscler Thromb Vasc Biol 32:1116–1123
Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AW, Bu G (2016) The role of APOE in cerebrovascular dysfunction. Acta Neuropathol 131:709–723
Acknowledgments
This work was supported by the National Institutes of Health (R01 AG055798, 1RF1 AG057933, and T32 AG061892).
Author information
Authors and Affiliations
Contributions
PC conceived the study, designed all figures and wrote the manuscript. EJK performed the experiments with help of EGDLC, MW, TW, and PC. JL provided rTg4510 breeders, and PMS provided APOE4 TR mice. PEC cloned APOE4, and DR packaged the AAV. TEG and DRB provided scientific input. EJK, EGDLC, TW, DRB and PC performed data analysis. All authors reviewed the manuscript and approved its final version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All animal experiments were approved by the University of Florida IACUC.
Consent for Publication
This manuscript has been approved for publication by all authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Figure S1
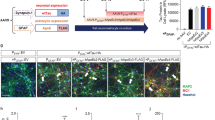
Analysis of APOE expression in rTg4510 mice expressing AAV-APOE4 and APOE TR mice. Soluble brain fractions of 4–6 month old APOE4 TR mice and 4 month old rTg4510 mice expressing AAV-APOE4 were probed with APOE antibody or actin antibody (A). Quantification of APOE protein band normalized to actin loading control (B). n = 4–5 mice/group. Unpaired t-test. ***p < 0.001. Representative images of 4 month old rTg4510 mouse brains, 4 month old rTg4510 mouse brains expressing AAV-APOE4 or 4 month old APOE TR brains co-immunostained with APOE and NeuN or APOE and GFAP antibodies (C-D). Scale bar: 200 μm; Zoom panel: 25 μm. Total counts of APOE/NeuN positive and APOE/GFAP positive cells from three different brain areas were averaged (E-F). n = 3 mice/group. Kruskal Wallis One way ANOVA with Dunn’s multiple comparisons test. *p < 0.05. Representative images from 4 month old control or AAV-APOE4 expressing rTg4510 mice showing co-immunostaining with ptau (CP13) and APOE antibodies (G). n = 3 mice/group. Scale Bar: 200 μm; Zoom panel: 25 μm. (PNG 943 kb)
Figure S2
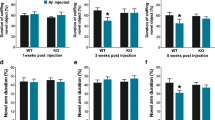
Analysis of AT8 immunoreactivity in rTg4510 mice. Representative images of 4 month old rTg4510 mouse brains (A) or 6 month old rTg4510 mouse brains (D) depicting AT8 ptau (pSer202/Thr205) immunoreactivity from the frontal cortex, motor cortex, auditory cortex, and hippocampal CA1 region. Scale bar: 100 μm. % AT8 immunoreactivity in the cortex (Ctx) and hippocampus (Hpc) is shown (B-C, E-F). n = 5–10 mice/group. Unpaired t test. Black symbols, male mice; gray symbols, female mice. (PNG 509 kb)
Figure S3
Analysis of tau inclusion and pretangle pathology in rTg4510 mice. Representative images of 4 month old rTg4510 mouse brains or 6 month old rTg4510 mouse brains immunostained with conformational tau antibodies MC1 (A-C, J-L), Alz50 (D-F, M-O) and inclusion pathology marker p62 (G-I, P-R) are shown. Scale Bar, 100 μm. Data is quantified from the cortex (Ctx) and hippocampus (Hpc) as % immunoreactivity. n = 5–10 mice/group. Unpaired t-test. *p < 0.05. Black symbols, male mice; gray symbols, female mice. (PNG 1232 kb)
Figure S4
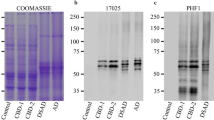
APOE levels in AAV-APOE4 transduced PS19 mice. Soluble brain fractions from 3 month old PS19 mice was probed with APOE antibody or Actin antibody (A). Quantification of APOE protein band normalized to actin loading control is shown (B). n = 4 mice/group. Unpaired t-test. ***p < 0.001. (PNG 78 kb)
Rights and permissions
About this article
Cite this article
Koller, E.J., Gonzalez De La Cruz, E., Weinrich, M. et al. Intracerebral Expression of AAV-APOE4 Is Not Sufficient to Alter Tau Burden in Two Distinct Models of Tauopathy. Mol Neurobiol 57, 1986–2001 (2020). https://doi.org/10.1007/s12035-019-01859-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01859-4