Abstract
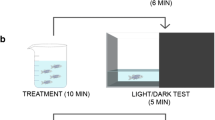
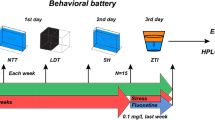
There is accumulating evidence on the use of N-acetylcysteine (NAC) in the treatment of patients with neuropsychiatric disorders. As a multi-target drug and a glutathione precursor, NAC is a promising molecule in the management of stress-related disorders, for which there is an expanding field of research investigating novel therapies targeting oxidative pathways. The deleterious effects of chronic stress in the central nervous system are a result of glutamatergic hyperactivation, glutathione (GSH) depletion, oxidative stress, and increased inflammatory response, among others. The aim of this study was to investigate the effects of NAC in zebrafish submitted to unpredictable chronic stress (UCS). Animals were initially stressed or not for 7 days, followed by treatment with NAC (1 mg/L, 10 min) or vehicle for 7 days. UCS decreased the number of entries and time spent in the top area in the novel tank test, which indicate increased anxiety levels. It also increased reactive oxygen species (ROS) levels and lipid peroxidation (TBARS) while decreased non-protein thiols (NPSH) and superoxide dismutase (SOD) activity. NAC reversed the anxiety-like behavior and oxidative damage observed in stressed animals. Additional studies are needed to investigate the effects of this agent on glutamatergic modulation and inflammatory markers related to stress. Nevertheless, our study adds to the existing body of evidence supporting the clinical evaluation of NAC in mood disorders, anxiety, post-traumatic stress disorder, and other conditions associated with stress.





Similar content being viewed by others
References
Bavarsad Shahripour R, Harrigan MR, Alexandrov AV (2014) N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav 4:108–122. https://doi.org/10.1002/brb3.208
Berk M, Malhi GS, Gray LJ, Dean OM (2013) The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci 34:167–177. https://doi.org/10.1016/j.tips.2013.01.001
Deepmala null, Slattery J, Kumar N et al (2015) Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev 55:294–321. https://doi.org/10.1016/j.neubiorev.2015.04.015
Fernandes BS, Dean OM, Dodd S, Malhi GS, Berk M (2016) N-acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry 77:e457–e466. https://doi.org/10.4088/JCP.15r09984
Minarini A, Ferrari S, Galletti M, Giambalvo N, Perrone D, Rioli G, Galeazzi GM (2017) N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol 13:279–292. https://doi.org/10.1080/17425255.2017.1251580
Dean O, Giorlando F, Berk M (2011) N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci 36:78–86. https://doi.org/10.1503/jpn.100057
Kowalczyk-Pachel D, Iciek M, Wydra K, Nowak E, Górny M, Filip M, Włodek L, Lorenc-Koci E (2016) Cysteine metabolism and oxidative processes in the rat liver and kidney after acute and repeated cocaine treatment. PLoS One 11:e0147238. https://doi.org/10.1371/journal.pone.0147238
Mocelin R, Marcon M, D’ambros S, Herrmann AP, da Rosa Araujo AS, Piato A (2017) Behavioral and biochemical effects of N-acetylcysteine in zebrafish acutely exposed to ethanol. Neurochem Res 43:458–464. https://doi.org/10.1007/s11064-017-2442-2
Mocelin R, Herrmann AP, Marcon M, Rambo CL, Rohden A, Bevilaqua F, de Abreu MS, Zanatta L et al (2015) N-acetylcysteine prevents stress-induced anxiety behavior in zebrafish. Pharmacol Biochem Behav 139:121–126. https://doi.org/10.1016/j.pbb.2015.08.006
Santos P, Herrmann AP, Benvenutti R, Noetzold G, Giongo F, Gama CS, Piato AL, Elisabetsky E (2017) Anxiolytic properties of N-acetylcysteine in mice. Behav Brain Res 317:461–469. https://doi.org/10.1016/j.bbr.2016.10.010
Schneider R, Bandiera S, Souza DG, Bellaver B, Caletti G, Quincozes-Santos A, Elisabetsky E, Gomez R (2017) N-acetylcysteine prevents alcohol related neuroinflammation in rats. Neurochem Res 42:2135–2141. https://doi.org/10.1007/s11064-017-2218-8
Schneider R, Santos CF, Clarimundo V et al (2015) N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats. Alcohol 49:259–263. https://doi.org/10.1016/j.alcohol.2015.01.009
Herrmann AP, Benvenutti R, Pilz LK, Elisabetsky E (2014) N-acetylcysteine prevents increased amphetamine sensitivity in social isolation-reared mice. Schizophr Res 155:109–111. https://doi.org/10.1016/j.schres.2014.03.012
Berk M, Dean OM, Cotton SM, Jeavons S, Tanious M, Kohlmann K, Hewitt K, Moss K et al (2014) The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry 75:628–636. https://doi.org/10.4088/JCP.13m08454
de Andrade KQ, Moura FA, dos Santos JM, de Araújo O, de Farias Santos J, Goulart M (2015) Oxidative stress and inflammation in hepatic diseases: therapeutic possibilities of N-acetylcysteine. Int J Mol Sci 16:30269–30308. https://doi.org/10.3390/ijms161226225
Chen S, Ren Q, Zhang J, Ye Y, Zhang Z, Xu Y, Guo M, Ji H et al (2014) N-acetyl-L-cysteine protects against cadmium-induced neuronal apoptosis by inhibiting ROS-dependent activation of Akt/mTOR pathway in mouse brain. Neuropathol Appl Neurobiol 40:759–777. https://doi.org/10.1111/nan.12103
Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34. https://doi.org/10.1038/nri.2015.5
Chrousos GP (2009) Stress and disorders of the stress system. Nat Rev Endocrinol 5:374–381. https://doi.org/10.1038/nrendo.2009.106
Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. https://doi.org/10.1038/nm.4050
Musazzi L, Marrocco J (2016) The many faces of stress: implications for neuropsychiatric disorders. Neural Plast 2016:8389737–8389732. https://doi.org/10.1155/2016/8389737
Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M (2016) Oxidative stress in neurodegenerative diseases. Mol Neurobiol 53:4094–4125. https://doi.org/10.1007/s12035-015-9337-5
Park CS, Yang XW (2015) Probing the stress and depression circuits with a disease gene. eLife 4. https://doi.org/10.7554/eLife.10829
Popoli M, Yan Z, McEwen BS, Sanacora G (2011) The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13:22–37. https://doi.org/10.1038/nrn3138
Sandi C, Haller J (2015) Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 16:290–304. https://doi.org/10.1038/nrn3918
Cummings JL, Zhong K (2006) Treatments for behavioural disorders in neurodegenerative diseases: drug development strategies. Nat Rev Drug Discov 5:64–74. https://doi.org/10.1038/nrd1928
Song C, Liu B-P, Zhang Y-P, Peng Z, Wang JJ, Collier AD, Echevarria DJ, Savelieva KV et al (2017) Modeling consequences of prolonged strong unpredictable stress in zebrafish: complex effects on behavior and physiology. Prog Neuro-Psychopharmacol Biol Psychiatry 81:384–394. https://doi.org/10.1016/j.pnpbp.2017.08.021
Marcon M, Mocelin R, Benvenutti R, Costa T, Herrmann AP, de Oliveira DL, Koakoski G, Barcellos LJG et al (2018) Environmental enrichment modulates the response to chronic stress in zebrafish. J Exp Biol 221:jeb176735. https://doi.org/10.1242/jeb.176735
Marcon M, Herrmann AP, Mocelin R, Rambo CL, Koakoski G, Abreu MS, Conterato GMM, Kist LW et al (2016) Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology 233:3815–3824. https://doi.org/10.1007/s00213-016-4408-5
Piato ÂL, Capiotti KM, Tamborski AR, Oses JP, Barcellos LJG, Bogo MR, Lara DR, Vianna MR et al (2011) Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog Neuro-Psychopharmacol Biol Psychiatry 35:561–567. https://doi.org/10.1016/j.pnpbp.2010.12.018
Rambo CL, Mocelin R, Marcon M, Villanova D, Koakoski G, de Abreu MS, Oliveira TA, Barcellos LJG et al (2017) Gender differences in aggression and cortisol levels in zebrafish subjected to unpredictable chronic stress. Physiol Behav 171:50–54. https://doi.org/10.1016/j.physbeh.2016.12.032
Müller TE, Nunes SZ, Silveira A, Loro VL, Rosemberg DB (2017) Repeated ethanol exposure alters social behavior and oxidative stress parameters of zebrafish. Prog Neuro-Psychopharmacol Biol Psychiatry 79:105–111. https://doi.org/10.1016/j.pnpbp.2017.05.026
Kysil EV, Meshalkina DA, Frick EE, Echevarria DJ, Rosemberg DB, Maximino C, Lima MG, Abreu MS et al (2017) Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 14:197–208. https://doi.org/10.1089/zeb.2016.1415
Pittman J, Piato A (2017) Develo** zebrafish depression-related models. In: The rights and wrongs of zebrafish: Behavioral phenoty** of zebrafish. Springer, Cham, pp. 33–43
Collier AD, Kalueff AV, Echevarria DJ (2017) Zebrafish models of anxiety-like behaviors. In: The rights and wrongs of zebrafish: behavioral phenoty** of zebrafish. Springer, Cham, pp. 45–72
Bencan Z, Sledge D, Levin ED (2009) Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav 94:75–80. https://doi.org/10.1016/j.pbb.2009.07.009
Gawali NB, Bulani VD, Gursahani MS, Deshpande PS, Kothavade PS, Juvekar AR (2017) Agmatine attenuates chronic unpredictable mild stress-induced anxiety, depression-like behaviours and cognitive impairment by modulating nitrergic signalling pathway. Brain Res 1663:66–77. https://doi.org/10.1016/j.brainres.2017.03.004
Kumar B, Kuhad A, Chopra K (2011) Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: behavioral and biochemical evidences. Psychopharmacology 214:819–828. https://doi.org/10.1007/s00213-010-2094-2
Chen T, Wang R, Jiang W, Wang H, Xu A, Lu G, Ren Y, Xu Y et al (2016) Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing rho signaling. Inflammation 39:483–492. https://doi.org/10.1007/s10753-015-0272-4
Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E (2013) Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 38:1698–1708. https://doi.org/10.1016/j.psyneuen.2013.02.004
Biala G, Pekala K, Boguszewska-Czubara A, Michalak A, Kruk-Slomka M, Budzynska B (2017) Behavioral and biochemical interaction between nicotine and chronic unpredictable mild stress in mice. Mol Neurobiol 54:904–921. https://doi.org/10.1007/s12035-016-9701-0
Biala G, Pekala K, Boguszewska-Czubara A, Michalak A, Kruk-Slomka M, Grot K, Budzynska B (2017) Behavioral and biochemical impact of chronic unpredictable mild stress on the acquisition of nicotine conditioned place preference in rats. Mol Neurobiol 55:3270–3289. https://doi.org/10.1007/s12035-017-0585-4
Maes M, Galecki P, Chang YS, Berk M (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuro-Psychopharmacol Biol Psychiatry 35:676–692. https://doi.org/10.1016/j.pnpbp.2010.05.004
Ortiz GG, Pacheco Moisés FP, Mireles-Ramírez M et al (2017) Oxidative stress: love and hate history in central nervous system. Adv Protein Chem Struct Biol 108:1–31. https://doi.org/10.1016/bs.apcsb.2017.01.003
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:205–214. https://doi.org/10.1038/nrd1330
Slot JW, Geuze HJ, Freeman BA, Crapo JD (1986) Intracellular localization of the copper-zinc and manganese superoxide dismutases in rat liver parenchymal cells. Lab Investig J Tech Methods Pathol 55:363–371
Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5:51–66. https://doi.org/10.1038/nprot.2009.197
Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Fernández AP, Rodrigo J, Boscá L, Leza JC (2000) Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J Neurochem 74:785–791
MacMillan-Crow LA, Crow JP, Thompson JA (1998) Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry (Mosc) 37:1613–1622. https://doi.org/10.1021/bi971894b
Filipović D, Zlatković J, Pajović SB (2009) The effect of acute or/and chronic stress on the MnSOD protein expression in rat prefrontal cortex and hippocampus. Gen Physiol Biophys 28:53–61
Thakur T, Gulati K, Rai N, Ray A (2017) Experimental studies on possible regulatory role of nitric oxide on the differential effects of chronic predictable and unpredictable stress on adaptive immune responses. Int Immunopharmacol 50:236–242. https://doi.org/10.1016/j.intimp.2017.07.002
Keller JN, Kindy MS, Holtsberg FW, St. Clair DK, Yen HC, Germeyer A, Steiner SM, Bruce-Keller AJ et al (1998) Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci 18:687–697
Barreiro E, Sánchez D, Gáldiz JB, Hussain SN, Gea J, ENIGMA in COPD project (2005) N-acetylcysteine increases manganese superoxide dismutase activity in septic rat diaphragms. Eur Respir J 26:1032–1039. https://doi.org/10.1183/09031936.05.00003705
Hicdonmez T, Kanter M, Tiryaki M, Parsak T, Cobanoglu S (2006) Neuroprotective effects of N-acetylcysteine on experimental closed head trauma in rats. Neurochem Res 31:473–481. https://doi.org/10.1007/s11064-006-9040-z
Günther M, Davidsson J, Plantman S, Norgren S, Mathiesen T, Risling M (2015) Neuroprotective effects of N-acetylcysteine amide on experimental focal penetrating brain injury in rats. J Clin Neurosci Off J Neurosurg Soc Australas 22:1477–1483. https://doi.org/10.1016/j.jocn.2015.03.025
Mao G, Goswami M, Kalen AL, Goswami PC, Sarsour EH (2016) N-acetyl-L-cysteine increases MnSOD activity and enhances the recruitment of quiescent human fibroblasts to the proliferation cycle during wound healing. Mol Biol Rep 43:31–39. https://doi.org/10.1007/s11033-015-3935-1
Nagata K, Iwasaki Y, Yamada T, Yuba T, Kono K, Hosogi S, Ohsugi S, Kuwahara H et al (2007) Overexpression of manganese superoxide dismutase by N-acetylcysteine in hyperoxic lung injury. Respir Med 101:800–807. https://doi.org/10.1016/j.rmed.2006.07.017
Yang Y-Y, Lee K-C, Huang Y-T, Wang YW, Hou MC, Lee FY, Lin HC, Lee SD (2008) Effects of N-acetylcysteine administration in hepatic microcirculation of rats with biliary cirrhosis. J Hepatol 49:25–33. https://doi.org/10.1016/j.jhep.2008.02.012
Peterlik D, Flor PJ, Uschold-Schmidt N (2016) The emerging role of metabotropic glutamate receptors in the pathophysiology of chronic stress-related disorders. Curr Neuropharmacol 14:514–539
Cacciatore I, Cornacchia C, Pinnen F, Mollica A, di Stefano A (2010) Prodrug approach for increasing cellular glutathione levels. Mol Basel Switz 15:1242–1264. https://doi.org/10.3390/molecules15031242
Acknowledgements
R.M and M.M are recipients of fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq, Proc. 401162/2016-8 and 302800/2017-4) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, Proc. 17/2551-0000974-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All protocols were approved by the Ethics Committee of Federal University of Rio Grande do Sul (process number #30914).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mocelin, R., Marcon, M., D’ambros, S. et al. N-Acetylcysteine Reverses Anxiety and Oxidative Damage Induced by Unpredictable Chronic Stress in Zebrafish. Mol Neurobiol 56, 1188–1195 (2019). https://doi.org/10.1007/s12035-018-1165-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1165-y