Abstract
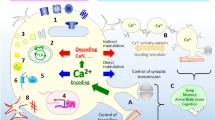
Astrocytes constitute a very abundant cell type in the mammalian central nervous system and play critical roles in brain function. During development, astrocytes are generated from neural progenitor cells only after these cells have generated neurons. This so called gliogenic switch is tightly regulated by intrinsic factors that inhibit the generation of astrocytes during the neurogenic period. Once neural progenitors acquire gliogenic competence, they differentiate into astrocytes in response to specific extracellular signals. Some of these signals are delivered by neurotrophic cytokines via activation of the gp130–JAK–signal transducer and activator of transcription system, whereas others depend on the activity of pituitary adenylate cyclase-activating polypeptide (PACAP) on specific PAC1 receptors that stimulate the production of cAMP. This results in the activation of the small GTPases Rap1 and Ras, and in the cAMP-dependent entry of extracellular calcium into the cell. Calcium, in turn, stimulates the transcription factor downstream regulatory element antagonist modulator (DREAM), which is bound to specific sites of the promoter of the glial fibrillary acidic protein gene, stimulating its expression during astrocyte differentiation. Lack of DREAM in vivo results in alterations in the number of neurons and astrocytes generated during development. Thus, the PACAP–cAMP–Ca2+–DREAM signaling cascade constitutes an important pathway to activate glial-specific gene expression during astrocyte differentiation.


Similar content being viewed by others
References
Bass NH, Hess HH, Pope A, Thalheimer C (1971) Quantitative cytoarchitectonic distribution of neurons, glia, and DNA in rat cerebral cortex. J Comp Neurol 143:481–490
Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci 26:523–530
Barres BA (2008) The mistery and magic of glia: A perspective on their roles in health and disease. Neuron 60:430–440
Theodosis DT, Poulain DA, Oliet SHR (2008) Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev 88:983–1008
Haydon PG, Carmignoto G (2006) Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86:1009–1031
Perea G, Araque A (2007) Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317:1083–1086
Ashner M, Allen JW, Kimelberg HK, LoPachin RM, Streit WJ (1999) Glial cells in neurotoxicity development. Annu Rev Pharmacol Toxicol 39:151–173
Maragakis NJ, Rothstein JD (2004) Glutamate transporters: animal models to neurologic disease. Neurobiol Dis 15:461–473
Gash DM, Zhang Z, Gerhardt G (1998) Neuroprotective and neurorestorative properties of GDNF. Ann Neurol 44:121–125
Blondel O, Collin C, McCarran WJ, Zhu S, Zamostiano R, Gozes I, Brenneman DE, McKay RDG (2000) A glia-derived signal regulating neuronal differentiation. J Neurosci 20:8012–8020
Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21:7153–7160
Araque A (2006) Astrocyte-neuron signaling in the brain. Implications for disease. Curr Opin Investig Drugs 7:619–624
Chen G, Li HM, Chen YR, Gu XS, Duan S (2007) Decreased estradiol release from astrocytes contributes to the neurodegeneration in a mouse model of Niemann-Pick type C. Glia 15:1509–1518
Lepore AL, Rauk B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ (2008) Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci 11:1294–1301
Quinlan RA, Brenner M, Goldman JE, Messing A (2007) GFAP and its role in Alexander disease. Exp Cell Res 10:2077–2087
Pinto L, Gotz M (2007) Radial glial cell heterogeneity—The source of diverse progeny in the CNS. Prog Neurobiol 83:2–23
Fan G, Martinowich K, Chin MH, He F, Fouse SD, Hutnik L, Hattori D, Ge W, Shen Y, Wu H, Ten Hoeve J, Shuai K, Sun YE (2005) DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 132:3345–3356
Namihira M, Nakashima K, Taga T (2004) Developmental stage-dependent regulation of DNA methylation and chromatin modification in an immature astrocyte-specific gene promoter. FEBS Lett 572:184–188
Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M, Taga T (2001) DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell 1:749–758
Song MR, Ghosh A (2004) FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat Neurosci 7:229–235
Angelastro JM, Mason J, Ignatova TN, Kukekov VG, Stengren GB, Goldman JE, Greene LA (2005) Downregulation of activating transcription factor 5 is required for differentiation of neural progenitor cells into astrocytes. J Neurosci 25:3889–3899
Hermanson O, Jepsen K, Rosenfeld MG (2002) N-CoR controls differentiation of neural stem cells into astrocytes. Nature 419:934–939
Cai L, Morrow EM, Cepko CL (2000) Misexpression of basic helix–loop–helix genes in the murine cerebral cortex affects cell fate choices and neuronal survival. Development 127:3021–3030
Nieto M, Schuurmans C, Britz O, Guillemot F (2001) Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron 29:401–413
Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, Greenberg ME (2001) Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104:365–376
Qian X, Davis AA, Goderie SK, Temple S (1997) FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron 18:81–93
Viti J, Feathers A, Phillips J, Lillien L (2003) Epidermal growth factor receptors control competence to interpret leukemia inhibitory factor as an astrocyte inducer in develo** cortex. J Neurosci 23:3385–3393
Barnabé-Heider F, Wasylnka JA, Fernandes KJL, Porsche C, Sendtner M, Kaplan DR, Miller FD (2005) Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 48:253–265
Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME (1997) Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 278:477–483
Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RDG (1996) Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev 10:3129–3140
Rajan P, McKay RDG (1998) Multiple routes to astrocytic differentiation in the CNS. J Neurosci 18:3620–3629
Schindler C, Levy DE, Decker T (2007) JAK-STAT signaling: From interferons to cytokines. J Biol Chem 282:20059–20063
Bugga L, Gadient RA, Kwan K, Stewart CL, Patterson PH (1998) Analysis of neuronal and glial phenotypes in brains of mice deficient in leukemia inhibitory factor. J Neurobiol 36:509–524
Koblar SA, Turnley AM, Classon BJ, Reid KL, Ware CB, Cheema SS, Murphy M, Bartlett PF (1998) Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. Proc Natl Acad Sci U S A 95:3178–3181
Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T (1999b) Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci 19:5429–5434
Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA (1996) Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17:595–606
Mabie PC, Mehler MF, Kessler JA (1999) Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype. J Neurosci 19:7077–7088
Mehler MF, Mabie PC, Zhang D, Kessler JA (1997) Bone morphogenetic proteins in the nervous system. Trends Neurosci 20:309–317
Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T (1999a) Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284:479–482
Cebolla B, Fernández-Pérez A, Perea G, Araque A, Vallejo M (2008) DREAM mediates cAMP-dependent, Ca2+-induced stimulation of GFAP gene expression and regulates cortical astrogliogenesis. J Neurosci 28:6703–6713
McManus M, Chen LC, Vallejo I, Vallejo M (1999) Astroglial differentiation of cortical precursor cells triggered by activation of the cAMP-dependent signaling pathway. J Neurosci 19:9004–9015
Vallejo I, Vallejo M (2002) Pituitary adenylate cyclase-activating polypeptide induces astrocyte differentiation of precursor cells from develo** cerebral cortex. Mol Cell Neurosci 21:671–683
Lastres-Becker I, Fernández-Pérez A, Cebolla B, Vallejo M (2008) Pituitary adenylate cyclase-activating polypeptide stimulates glial fibrillary acidic protein gene expression in cortical precursor cells by activating Ras and Rap1. Mol Cell Neurosci 39:291–301
Cameron HA, Hazel TG, McKay RD (1998) Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol 36:287–306
Levitt P, Harvey JA, Simansky K, Murphy EH (1997) New evidence for neurotransmitter influences on brain development. Trends Neurosci 20:269–274
Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA (1998) International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev 50:265–270
Nicot A, DiCicco-Bloom E (2001) Regulation of neuroblast mitosis is determined by PACAP receptor isoform expression. Proc Natl Acad Sci U S A 98:4758–4763
Spengler D, Waeber C, Pantaloni C, Florian H, Bockaert J, Seeburg PH, Journot L (1993) Differential signal transduction by five splice variants of the PACAP receptor. Nature 365:170–175
Chatterjee TK, Sharma RV, Fisher RA (1996) Molecular cloning of a novel variant of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor that stimulates calcium influx by activation of L-type calcium channels. J Biol Chem 217:32226–32232
Park JK, Williams BP, Alberta JA, Stiles CD (1999) Bipotent cortical progenitor cells process conflicting cues for neurons and glia in a hierarchical manner. J Neurosci 19:10383–10389
Williams BP, Park JK, Alberta JA, Muhlebach SG, Hwang GY, Roberts TM, Stiles CD (1997) A PDGF-regulated immediate early gene response initiates neuronal differentiation in ventricular zone progenitor cells. Neuron 18:553–552
Watanabe J, Ohba M, Ohno F, Kikuyama S, Nakamura M, Nakaya K, Arimura A, Shioda S, Nakajo S (2006) Pituitary adenylate cyclase-activating polypeptide-induced differentiation of embryonic neural stem cells into astrocytes is mediated via the b isoform of protein kinase C. J Neurosci Res 84:1645–1655
Ohno F, Watanabe J, Sekihara H, Hirabayashi T, Arata S, Kikuyama S, Shioda S, Nakaya K, Nakajo S (2005) Pituitary adenylate cyclase-activating polypeptide promotes differentiation of mouse neural stem cells into astrocytes. Regul Pept 126:115–122
Nishimoto M, Furuta A, Aoki S, Kudo Y, Miyakawa H, Wada K (2007) PACAP/PAC1 autocrine system promotes proliferation and astrogenesis in neural progenitor cells. Glia 55:317–327
Suh J, Lu N, Nicot A, Tatsuno I, DiCicco-Bloom E (2001) PACAP is an anti-mitogenic signal in develo** cerebral cortex. Nat Neurosci 4:123–124
Watanabe J, Nakamachi T, Matsuo R, Hayashi D, Nakamura M, Kikuyama S, Nakago S, Shioda S (2007) Localization, characterization and function of pituitary adenylate cyclase-activating polypeptide during brain developent. Peptides 28:1713–1719
Cebolla B, Vallejo M (2006) Nuclear factor-I regulates glial fibrillary acidic protein gene expression in astrocytes differentiated from cortical precursor cells. J Neurochem 97:1057–1070
Zupan V, Hill JM, Brenneman DE, Gozes I, Fridkin M, Roberecht P, Evrard P, Gressens P (1998) Involvement of pituitary adenylate cyclase-activating polypeptide II vasoactive intestinal peptide 2 receptor in mouse neocortical astrocytogenesis. J Neurochem 70:2165–2173
Carey RG, Li B, DiCicco-Bloom E (2002) Pituitary adenylate cyclase activating polypeptide anti-mitogenic signaling in cerebral cortical progenitors is regulated by p57Kip2-dependent CDK2 activity. J Neurosci 22:1583–1591
Cazillis M, Gonzalez BJ, Billardon C, Lombet A, Fraichard A, Samarut J, Gressens P, Vaudry H, Rostene W (2004) VIP and PACAP induce selective neuronal differentiation of mouse embryonic stem cells. Eur J Neurosci 19:798–808
Lee M, Lelievre V, Zhao P, Torres M, Rodriguez W, Byun JY, Doshi S, Ioffe Y, Gupta G, de los Monteros AE, de Vellis J, Waschek J (2001) Pituitary adenylyl cyclase-activating polypeptide stimulates DNA synthesis but delays maturation of oligodendrocyte progenitors. J Neurosci 21:3849–3859
Lelievre V, Hu Z, Byun JY, Ioffe Y, Waschek JA (2002) Fibroblast growth factor-2 converts PACAP growth action on embryonic hindbrain precursors from stimulation to inhibition. J Neurosci Res 67:566–573
Pham N, Cheglakov I, Koch CA, de Hoog CL, Moran MF, Rotin D (2000) The guanine nucleotide exchange factor CNrasGEF activates Ras in response to cAMP and cGMP. Curr Biol 10:555–558
Bos JL (2003) Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4:733–738
Bouschet T, Perez V, Fernandez C, Bockaert J, Eychene A, Journot L (2003) Stimulation of the ERK pathway by GTP-loaded Rap1 requires the concomitant activation of Ras, protein kinase C, and protein kinase A in neuronal cells. J Biol Chem 278:4778–4785
Dasgupta B, Dugan LL, Gutmann DH (2003) The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J Neurosci 23:8948–8954
Obara Y, Horgan A, Stork PJS (2007) The requirement of Ras and Rap1 for the activation of ERKs by cAMP, PACAP, and KCl in cerebellar granule cells. J Neurochem 101:470–482
Romano D, Magalon K, Ciampini A, Talet C, Enjalbert A, Gerard C (2003) Differential involvement of the Ras and Rap1 small GTPases in vasoactive intestinal and pituitary adenylyl cyclase activating polypeptides control of the prolactin gene. J Biol Chem 278:51386–51394
Matallanas D, Arozarena I, Berciano M, Aaronson DS, Pellicer A, Lafarga M, Crespo P (2003) Differences on the inhibitory specificities of H-Ras, K-Ras, and N-Ras (N17) dominant negative mutants are related to their membrane microlocalization. J Biol Chem 278:4572–4581
Vossler MR, Yao H, York R, Pan MG, Rim CS, Stork PJS (1997) cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-Dependent pathway. Cell 89:73–82
Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4:901–906
Apicelli AJ, Uhlmann EJ, Baldwin RL, Dong D, Nagy A, Guha A, Gutman DH (2003) Role of the Rap1 GTPase in astrocyte growth regulation. Glia 42:225–234
Barnett SC, Rosario M, Doyle A, Kilbey A, Lovatt A, Gillespie DAF (1995) Differential regulation of AP-1 and novel TRE-specific DNA-binding complexes during differentiation of oligodendrocyte-type-2-astrocyte (O-2A) progenitor cells. Development 121:3969–3977
Kahn MA, Huang CJ, Caruso A, Barresi V, Nazarian R, Condorelli DF, De Vellis J (1997) Ciliary neurotrophic factor activates JAK/Stat signal transduction cascade and induces transcriptional expression of glial fibrillary acidic protein in glial cells. J Neurochem 68:1413–1423
Krohn K, Rozovsky I, Wals P, Teter B, Anderson CP, Finch CE (1999) Glial fibrillary acidic protein transcription responses to transforming growth factor-b1 and interleukin-1b are mediated by a nuclear factor-1-like site in the near-upstream promoter. J Neurochem 72:1353–1361
Lee Y, Su M, Messing A, Brenner M (2006) Astrocyte heterogeneity revealed by expression of a GFAP-LacZ transgene. Glia 53:677–687
Lee Y, Messing A, Su M, Brenner M (2008) GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56:481–493
Rajan P, Panchision DM, Newell LF, McKay RDG (2003) BMPs signal alternately through a SMAD or FRAP-STAT pathway to regulate fate choice in CNS stem cells. J Cell Biol 161:911–921
Stone DJ, Song Y, Anderson CP, Krohn KK, Finch CE, Rozovsky I (1998) Bidirectional transcription regulation of glial fibrillary acidic protein by estradiol in vivo and in vitro. Endocrinology 139:3202–3209
Deneen B, Ho R, Lucaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ (2006) The transcription factor NFIA controls the onset of gliogenesis in the develo** spinal cord. Neuron 52:953–968
Gopalan SM, Wilczynska KM, Konik BS, Bryan L, Kordula T (2006) Nuclear factor-I-X regulates astrocyte-specific expression of the alpha1-antichymotrypsin and glial fibrillary acidic protein genes. J Biol Chem 281:13126–13133
Gronostajski RM (2000) Roles of the NF1/CTF gene family in transcription and development. Gene 249:31–45
Das Neves G, Duchala CS, Tolentino-Silva F, Haxhiu MA, Colmenares C, Macklin WB, Campbell CE, Butz KG, Gronostajski RM (1999) Disruption of the murine nuclear factor I-A gene (Nfia) results in perinatal lethality, hydrocephalus, and agenesis of the corpus callosum. Proc Natl Acad Sci U S A 96:11946–11951
Shu T, Butz KG, Plachez C, Gronostajski RM, Richards LJ (2003) Abnormal development of forebrain midline glia and commissural projections in Nfia knock-out mice. J Neurosci 23:203–212
Steele-Perkins G, Plachez C, Butz KG, Yang G, Bachurski CJ, Kinsman SL, Litwack ED, Richards LJ, Gronostajski RM (2005) The transcription factor gene Nfib is essential for both lung maturation and brain development. Mol Cell Biol 25:685
Belikov S, Holmqvist PH, Astrand C, Wrange O (2004) Nuclear factor 1 and octamer transcription factor 1 binding preset the chromatin structure of the mouse mammary tumor virus promoter for hormone induction. J Biol Chem 279:49857–49867
Hebbar PB, Archer TK (2007) Chromatin-dependent cooperativity between site-specific transcription factors in vivo. J Biol Chem 282:8284–8291
Mukhopadhyay SS, Wyszomierski SL, Gronostajski RM, Rosen JM (2001) Differential interactions of specific nuclear factor I isoforms with the glucocorticoid receptor and STAT5 in the cooperative regulation of WAP gene transcription. Mol Cell Biol 21:6859–6869
Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR (1999) DREAM is a Ca2+-regulated transcriptional repressor. Nature 398:80–84
Ledo F, Carrion AM, Link WA, Mellstrom B, Naranjo JR (2000) DREAM-alphaCREM interaction via leucine-charged domains derepresses downstream regulatory element-dependent transcription. Mol Cell Biol 20:9120–9126
Liu Z, Geng L, Li R, He X, Zheng JQ, **e Z (2003) Frequency modulation of synchronized Ca2+ spikes in cultured hippocampal networks through G-protein-coupled receptors. J Neurosci 23:4156–4163
Morita K, Sakakibara A, Kitayama S, Kumagai K, Tanne K, Dohi T (2002) Pituitary adenylate cyclase-activating polypeptide induces a sustained increase in intracellular free Ca2+ concentration and catecholamine release by activating Ca2+ influx via receptor-stimulated Ca2+ entry, Independent of store-operated Ca2+ channels, and voltage-dependent Ca2+ channels in bovine adrenal medullary chromaffin cells. J Pharmacol Exp Ther 302:972–982
Osipenko ON, Barrie A, Allen JM, Gurney AM (2000) Pituitary adenylate cyclase-activating peptide activates multiple intracellular signaling pathways to regulate ion channels in PC12 cells. J Biol Chem 275:16626–16631
Payet M, Bilodeau L, Breault L, Fournier A, Yon L, Vaudry H, Gallo-Payet N (2003) PAC1 receptor activation by PACAP-38 mediates Ca2+ release from a cAMP-dependent pool in human fetal adrenal gland chromaffin cells. J Biol Chem 278:1663–1670
Przywara DA, Guo X, Angelilli ML, Wakade TD, Wakade AR (1996) A non-cholinergic transmitter, pituitary adenylate cyclase-activating polypeptide, utilizes a novel mechanism to evoke catecholamine secretion in rat adrenal chromaffin cells. J Biol Chem 271:10545–10550
Osawa M, Dace A, Tong KI, Valiveti A, Ikura M, Ames JB (2005) Mg2+ and Ca2+ differentially regulate DNA binding and dimerization of DREAM. J Biol Chem 280:18008–18014
Osawa M, Tong KI, Lilliehook C, Wasco W, Buxbaum JD, Cheng HYM, Penninger JM, Ikura M, Ames JB (2001) Calcium-regulated DNA binding and oligomerization of the neuronal calcium-sensing protein, calsenilin/DREAM/KChIP3. J Biol Chem 276:41005–41013
Lusin JD, Vanarotti M, Li C, Valiveti A, Ames JB (2008) NMR structure of DREAM: Implications for Ca(2+)-dependent DNA binding and protein dimerization. Biochemistry 47:2252–2264
Gomez-Villafuertes R, Torres B, Barrio J, Savignac M, Gabellimi N, Rizzato F, Pintado B, Gurtierrez-Adan A, Mellstrom B, Carafoli E, Naranjo JR (2005) Downstream regulatory element antagonist modulator regulates Ca2+ homeostasis and viability in cerebellar neurons. J Neurosci 25:10822–10830
Rivas M, Mellstrom B, Naranjo JR, Santisteban P (2004) Transcriptional repressor DREAM interacts with thyroid transcription factor-1 and regulates thyroglobulin gene expression. J Biol Chem 279:33114–33122
Scsucova S, Palacios D, Savignac M, Mellstrom B, Naranjo JR, Aranda A (2005) The repressor DREAM acts as a transcriptional activator on vitamin D and retinoic acid response elements. Nucl Acids Res 33:2269–2279
Miller FD, Gauthier AS (2007) Timing is everything: Making neurons versus glia in the develo** cortex. Neuron 54:357–369
Acknowledgements
I would like to thank past and present members of my laboratory who generated published data discussed in this review and Javier Perez for electronically drawing the figures. Research funding was provided by grants form the Spanish Ministry of Science and Innovation and from the Comunidad de Madrid.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vallejo, M. PACAP signaling to DREAM: A cAMP-Dependent Pathway that Regulates Cortical Astrogliogenesis. Mol Neurobiol 39, 90–100 (2009). https://doi.org/10.1007/s12035-009-8055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-009-8055-2