Abstract
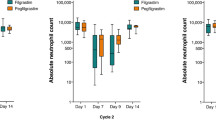
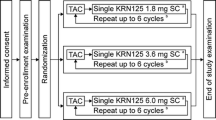
A chemotherapy regimen of docetaxel, doxorubicin and cyclophosphamide (TAC) has been accepted as a standard care because of their superior clinical benefit in early-stage breast cancer patients, but with a higher risk of neutropenia. Pegfilgrastim is a once-per-cycle therapy for prophylactic neutrophil support and neutropenia prevention. There was still a lack of direct evidences for finding an optimal fixed dose of pegfilgrastim in Chinese breast cancer patients receiving TAC regimen. An open-label, randomized, phase II study was designed to compare the effects of pegfilgrastim with filgrastim. Eighteen centers in China enrolled 171 eligible female breast cancer patients with cycles of TAC chemotherapy treatment, randomized into four arms, received a single subcutaneous injection of pegfilgrastim (60, 100 or 120 µg/kg) per chemotherapy cycle or daily subcutaneous injections of filgrastim 5 µg/kg 24 h after chemotherapy. Efficacy and safety were analyzed. In ITT population, the mean duration of grade 3+ neutropenia (neutrophil count <1.0 × 109/l) was 2.09, 1.53 and 1.73 days in patients who received pegfilgrastim 60, 100 and 120 µg/kg/cycle, respectively, and 1.69 days in patients who received 5 µg/kg/day filgrastim (P = 0.043). The incidence of grade 3+ neutropenia was 76, 83 and 74 % for doses of pegfilgrastim and 90 % for filgrastim (P = 0.409). The results for febrile neutropenia, time to neutrophil recovery and neutrophil profile were also not significantly different between arms. The safety profiles of pegfilgrastim and filgrastim were similar. A single dose of 100 µg/kg once-per-cycle administration of pegfilgrastim provided neutrophil support and a safety profile comparable to daily subcutaneous injections of filgrastim in Chinese breast cancer patients receiving TAC chemotherapy.



Similar content being viewed by others
References
De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53.
Early Breast Cancer Trialists’ Collaborative Group et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomized trials. Lancet. 2012;379:432–44.
Mackey JR, Martin M, Pienkowski T, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomized BCIRG 001 trial. Lancet Oncol. 2013;14:72–80.
WolfgangE TadeuszP, John C, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2–normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol. 2011;29:3877–84.
Trudeau Maureen, Charbonneau Flay, Gelmon Karen, et al. Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol. 2005;6:886–98.
Von Minckwitz G, Blohmer JU, Raab G, et al. In vivo chemosensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol. 2005;16:56–63.
Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–76.
Citron ML, Berry DA, Cirrincione C, Hudis C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of intergroup trial C9741/cancer and leukemia group B trial 9741. J Clin Oncol. 2003;21:1431–9.
Early Breast Cancer Trialists’ Collaborative Group. (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–717.
Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13.
Martín Miguel, Seguí Miguel A, Antón Antonio, et al. Adjuvant docetaxel for high-risk, node negative breast cancer. N Engl J Med. 2010;363:2200–10.
Martin M, Lluch A, Segui MA, et al. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol. 2006;17:1205–12.
Von Minckwitz G, Blohmer JU, Gerber B, et al. Primary prophylaxis with 3 weekly pegfilgrastim and ciprofloxacin effectively prevent (febrile) neutropenia and infection during neoadjuvant chemotherapy with docetaxel/doxorubicin/cyclophosphamide (TAC)in breast cancer patients. J Clin Oncol. 2005;23:8008.
Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2011;47:8–32.
Morstyn G, Campbell L, Souza LM, Alton NK, Keech J, Green M, Sheridan W, Metcalf D, Fox R. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988;1:667.
Morstyn G, Campbell L, Lieschke G, Layton JE, Maher D, O’Connor M, Green M, Sheridan W, Vincent M, Alton K, Souza L, McGrath K, Fox RM. Treatment of chemotherapy-induced neutropenia by subcutaneously administered granulocyte colony-stimulating factor with optimization of dose and duration of therapy. J Clin Oncol. 1989;7:1554.
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205.
Zamboni WC. Pharmacokinetics of pegfilgrastim. Pharmacotherapy. 2003;23(8 Pt 2):9S–14S.
Hershman Dawn L, Wilde Elizabeth T, et al. Uptake and economic impact of first-cycle colony-stimulating factor use during adjuvant treatment of breast cancer. J Clin Oncol. 2012;30:806–12.
Morstyn G, Campbell L, Souza LM, et al. Effects of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988;1:667–72.
Gabrilove JL, Jakubowski A, Scher H, et al. The effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N Engl J Med. 1988;318:1414–22.
Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–70.
Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy inpatients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20:727–31.
Holmes FA, Jones SE, O’Shaughnessy J, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13:903–9.
Green MD, Koelbl H, Baselga J, Galid A, Guillem V, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14:29–35.
Katy LC, Jason M, Sophie W, et al. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404.
Shi Yuan-Kai, Chen Qiang, et al. Pegylated filgrastim is comparable with filgrastim as support for commonly used chemotherapy regimens: a multicenter, randomized, crossover phase 3 study. Anticancer Drugs. 2013;24:641–7.
von Minckwitz G, Kummel S, du Bois A, et al. Pegfilgrastim ± ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol. 2008;19:292–8.
Park KH, Sohn JH, Lee S, et al. A randomized, multi-center, open-label, phase II study of once-per-cycle DA-3031, a biosimilar pegylated G-CSF, compared with daily filgrastim in patients receiving TAC chemotherapy for early-stage breast cancer. Invest New Drugs. 2013;31:1300–6.
Acknowledgments
This work was partially supported by Research Grants 81473069 and 81273176 from the National Natural Science Foundation of China.
Conflict of interest
We declare that we have no conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wei Zhang and Zhiwei Jiang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, W., Jiang, Z., Wang, L. et al. An open-label, randomized, multicenter dose-finding study of once-per-cycle pegfilgrastim versus daily filgrastim in Chinese breast cancer patients receiving TAC chemotherapy. Med Oncol 32, 147 (2015). https://doi.org/10.1007/s12032-015-0537-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0537-7