Abstract
Background
Seizures are a harmful complication of acute intracerebral hemorrhage (ICH). “Early” seizures in the first week after ICH are a risk factor for deterioration, later seizures, and herniation. Ideally, seizure medications after ICH would only be administered to patients with a high likelihood to have seizures. We developed and validated machine learning (ML) models to predict early seizures after ICH.
Methods
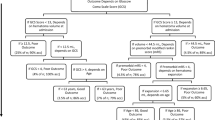
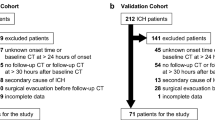
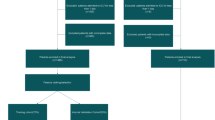
We used two large datasets to train and then validate our models in an entirely independent test set. The first model (“CAV”) predicted early seizures from a subset of variables of the CAVE score (a prediction rule for later seizures)—cortical hematoma location, age less than 65 years, and hematoma volume greater than 10 mL—whereas early seizure was the dependent variable. We attempted to improve on the “CAV” model by adding anticoagulant use, antiplatelet use, Glasgow Coma Scale, international normalized ratio, and systolic blood pressure (“CAV + ”). For each model we used logistic regression, lasso regression, support vector machines, boosted trees (Xgboost), and random forest models. Final model performance was reported as the area under the receiver operating characteristic curve (AUC) using receiver operating characteristic models for the test data. The setting of the study was two large academic institutions: institution 1, 634 patients; institution 2, 230 patients. There were no interventions.
Results
Early seizures were predicted across the ML models by the CAV score in test data, (AUC 0.72, 95% confidence interval 0.62–0.82). The ML model that predicted early seizure better in the test data was Xgboost (AUC 0.79, 95% confidence interval 0.71–0.87, p = 0.04) compared with the CAV model AUC.
Conclusions
Early seizures after ICH are predictable. Models using cortical hematoma location, age less than 65 years, and hematoma volume greater than 10 mL had a good accuracy rate, and performance improved with more independent variables. Additional methods to predict seizures could improve patient selection for monitoring and prophylactic seizure medications.

Similar content being viewed by others
Data Availability
Anonymized data and associated documentation will be made available by request from any qualified investigator for the purposes of reproducing the analysis.
References
Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254-743.
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–60.
Sheikh ZB, Stretz C, Maciel CB, et al. Deep versus lobar intraparenchymal hemorrhage: seizures, hyperexcitable patterns, and clinical outcomes. Crit Care Med. 2020;48:e505–13.
Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441–6.
Naidech AM, Weaver B, Maas M, Bleck TP, VanHaerents S, Schuele SU. Early seizures are predictive of worse health-related quality of life at follow-up after intracerebral hemorrhage. Crit Care Med. 2021;49:e578–84.
Vespa PM, Olson DM, John S, et al. Evaluating the clinical impact of rapid response electroencephalography: the DECIDE multicenter prospective observational clinical study. Crit Care Med. 2020;48:1249–57.
Haapaniemi E, Strbian D, Rossi C, et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke. 2014;45:1971–6.
Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke. 2009;40:3810–5.
Naidech AM, Beaumont J, Muldoon K, et al. Prophylactic seizure medication and health-related quality of life after intracerebral hemorrhage. Crit Care Med. 2018;46:1480–5.
Messe SR, Sansing LH, Cucchiara BL, et al. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care. 2009;11:38–44.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60.
Faigle R, Marsh EB, Llinas RH, Urrutia VC, Gottesman RF. Novel score predicting gastrostomy tube placement in intracerebral hemorrhage. Stroke. 2015;46:31–6.
Naidech AM, Beaumont JL, Berman M, et al. Dichotomous “good outcome” indicates mobility more than cognitive or social quality of life. Crit Care Med. 2015;43:1654–9.
Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4.
De Herdt V, Dumont F, Hénon H, et al. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology. 2011;77:1794–800.
Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med. 2010;77:791–9.
Yildiz OK, Arsava EM, Akpinar E, Topcuoglu MA. Previous antiplatelet use is associated with hematoma expansion in patients with spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2012;21:760–6.
Burchell SR, Tang J, Zhang JH. Hematoma expansion following intracerebral hemorrhage: mechanisms targeting the coagulation cascade and platelet activation. Curr Drug Targets. 2017;18:1329–44.
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–7.
Sakamoto Y, Koga M, Todo K, et al. Relative systolic blood pressure reduction and clinical outcomes in hyperacute intracerebral hemorrhage: the SAMURAI-ICH observational study. J Hypertens. 2015;33:1069–73.
Maas MB, Nemeth AJ, Rosenberg NF, et al. Subarachnoid extension of primary intracerebral hemorrhage is associated with poor outcomes. Stroke. 2013;44:653–7.
James G, Witten D, Hastie T, Tibshirani R. An introduction to statistical learning with applications in R. New York: Springer; 2013.
Chen T, Guestrin C. Xgboost: a scalable tree boosting system. 22nd ACM SIGKDD International Conference on Knowledge discovery and data mining. 2016;785–94.
Breiman L. Random forests. Mach Learn. 2001;45:5–32.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Batuwita R, Palade V. Class imbalance learning methods for support vector machines. In: He H, Ma Y, editors. Imbalanced learning: foundations, algorithms, and applications. Piscataway: IEEE Press; 2013. p. 1–20.
Bolandzadeh N, Kording K, Salowitz N. Predicting cognitive function from clinical measures of physical function and health status in older adults. PLoS ONE. 2015;10:e0119075.
Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc B. 2005;67:301–20.
Hall AN, Weaver B, Liotta E, et al. Identifying modifiable predictors of patient outcomes after intracerebral hemorrhage with machine learning. Neurocrit Care. 2021;34:73–84.
Naidech AM, Beaumont JL, Rosenberg NF, et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am J Respir Crit Care Med. 2013;188:1331–7.
Naidech AM, Beaumont J, Jahromi B, Prabhakaran S, Kho A, Holl JL. Evolving use of seizure medications after intracerebral hemorrhage: a multicenter study. Neurology. 2017;88:52–6.
Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–65.
Nuwer M. Assessment of digital EEG, quantitative EEG, and EEG brain map**: report of the American Academy of neurology and the American clinical neurophysiology society. Neurology. 1997;49:277–92.
Struck AF, Ustun B, Ruiz AR, et al. Association of an electroencephalography-based risk score with seizure probability in hospitalized patients. JAMA Neurol. 2017;74:1419–24.
Acknowledgements
J.M. is supported in part by T32 LM012203, R.F. is supported by a K23 NS101124, and A.M.N. is supported in part by R01 NS110779 and U01 NS110772.
Author information
Authors and Affiliations
Contributions
Bunney and Murphy performed analysis and wrote and edited the article. Colton, Wang, and Shin edited the article. Faigle supplied data and edited the article. Naidech provided data, performed analysis, and edited the article. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article do not have any conflicts of interest to report.
Ethical Approval/Informed Consent
We confirm compliance with the ethical approval and informed consent standards for human studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bunney, G., Murphy, J., Colton, K. et al. Predicting Early Seizures After Intracerebral Hemorrhage with Machine Learning. Neurocrit Care 37 (Suppl 2), 322–327 (2022). https://doi.org/10.1007/s12028-022-01470-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01470-x