Abstract
Aim
To analyze if the 1mg-dexamethasone suppression test (DST) is a reliable marker of glucocorticoid excess and cardiometabolic risk in patients with adrenal incidentalomas (AIs).
Methods
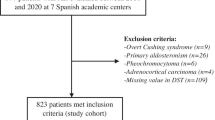
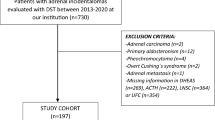
Cross-sectional study of patients with nonfunctioning adrenal incidentalomas (NFAIs, defined by cortisol post-DST ≤ 1.8 µg/dL) and patients with autonomous cortisol secretion (ACS, defined by cortisol post-DST > 1.8 µg/Dl). The urinary steroid profile (USP) was determined by gas chromatography coupled to mass spectrometry. Both groups were matched by sex, age and body mass index.
Results
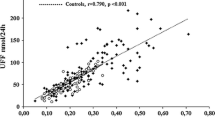
Forty-nine patients with AIs (25 with ACS and 24 with NFAI) were included. As a whole, AIs showed a high excretion of β-cortolone, tetrahydro-11-deoxycortisol (THS), α-cortolone, α-cortol, tetrahydrocortisol (THF) and tetrahydrocortisone (THE). A positive yet modest correlation between post-DST cortisol and total excretion of glucocorticoid metabolites (r = 0.401, P = 0.004) was observed, with the stronger being observed with total THS (r = 0.548, P < 0.001) and THF (r = 0.441, P = 0.002). Some of the metabolites that were elevated in patients with AIs, were higher in patients with ACS-related comorbidities than in those without comorbidities. Post-DST cortisol showed a fair diagnostic accuracy for the prediction of ACS-related comorbidities (AUC 0.767 [95% CI 0.634–0.882]). However, post-DST diagnostic accuracy improved when combined with urinary cortisone, α-cortol, THS and serum DHEAS (0.853 [0.712‒0.954]).
Conclusion
The DST has a positive, but modest, correlation with urinary glucocorticoid excretion. Similarly, the diagnostic accuracy of the DST for the prediction of ACS-related comorbidities is only fair, but it may be improved if combined with the results of the USP and serum DHEAS.
Significance statement
This is the first study aimed to evaluate if 1mg-dexamethasone suppression test (DST) is a reliable marker of glucocorticoid excess and cardiometabolic risk in patients with adrenal incidentalomas (AIs) and if urinary steroid profile was measured by GS-MS could improve such a prediction. We found a positive yet modest correlation between post-DST cortisol and total excretion of glucocorticoid metabolites, with the stronger being observed with total tetrahydro-11-deoxycortisol (THS) and tetrahydrocortisol. Post-DST cortisol showed a fair diagnostic accuracy for the prediction of ACS-related comorbidities (AUC 0.767). However, post-DST diagnostic accuracy improved when combined with urinary cortisone, α-cortol, THS and serum DHEAS (0.853).

Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- AIs:
-
adrenal incidentalomas
- ACS:
-
autonomous cortisol secretion
- BMI:
-
body mass index
- CV:
-
coefficient of variation
- DST:
-
dexamethasone suppression test
- 20α-DHF:
-
20α-dihydrocortisol
- 20β-DHF:
-
20β-dihydrocortisol
- DHEA:
-
Dehydroepiandrosterona
- FPG:
-
fasting plasma glucose
- GFR:
-
glomerular filtration rate
- NFAI:
-
non-functioning adrenal incidentalomas
- 17HP:
-
17-OH-Pregnanolone
- 5PD:
-
5-Pregnenediol
- 5PT:
-
5-Pregnenetriol
- PD:
-
Pregnanediol
- PT:
-
Pregnanetriol
- PTONE:
-
Pregnanetriolone
- UFC:
-
urinary free cortisol
- THF:
-
Tetrahydrocortisol
- THE:
-
Tetrahydrocortisone
- THS:
-
Tetrahydro-11-deoxycortisol
- THA:
-
Tetrahydro-11-dehydrocorticosterone
- 5 alfa-THA:
-
5α-Tetrahydro-11-dehydrocorticosterone
- THB:
-
Tetrahydrocortisone
- 5α-THB:
-
5α-Tetrahydrocorticosterone
- THAldo:
-
Tetrahydroaldosterone
- THDOC:
-
Tetrahydrodeoxycorticosterone
- 5 alfa-THDOC:
-
5α-Tetrahydrodesoxycorticosterone
- USP:
-
urinary steroid profile
References
I. Bancos, Adrenal incidentalomas: insights into prevalence. Ann. Intern. Med. 2022. https://doi.org/10.7326/m22-2600
G. Reimondo, E. Castellano, M. Grosso, R. Priotto, S. Puglisi, A. Pia et al. Adrenal incidentalomas are tied to increased risk of diabetes: findings from a prospective study. J. Clin. Endocrinol. Metab. 105, E973–E981 (2020). https://doi.org/10.1210/clinem/dgz284
M. Fassnacht, W. Arlt, I. Bancos, H. Dralle, J. Newell-Price, A. Sahdev et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 175, G1–G34 (2016). https://doi.org/10.1530/EJE-16-0467
M. Araujo-Castro, Cardiometabolic profile and urinary metabolomic alterations in non-functioning adrenal incidentalomas: a review. Clin. Endocrinol. 2022. https://doi.org/10.1111/cen.14745
A. Adamska, V. Ulychnyi, K. Siewko, A. Popławska-Kita, M. Szelachowska, M. Adamski, et al. Cardiovascular risk factors in mild adrenal autonomous cortisol secretion in a Caucasian population. Endocr. Connect. 2022;11. https://doi.org/10.1530/EC-22-0074
A. Kjellbom, O. Lindgren, S. Puvaneswaralingam, M. Löndahl, H. Olsen, Association between mortality and levels of autonomous cortisol secretion by adrenal incidentalomas. Ann. Intern. Med. 174, 1041–1049 (2021). https://doi.org/10.7326/M20-7946
M. Araujo-Castro, A. García Cano, L. Jiménez Mendiguchía, H.F. Escobar-Morreale, P. Valderrábano, Diagnostic accuracy of the different hormonal tests used for the diagnosis of autonomous cortisol secretion. Sci. Rep. 2021;11. https://doi.org/10.1038/s41598-021-00011-4
M. Araujo-Castro, P.P. Ramírez, C.R. Lázaro, R.G. Centeno, P.G. Gimeno, M.T. Fernández-Ladreda et al. Accuracy of the dexamethasone suppression test for the prediction of autonomous cortisol secretion-related comorbidities in adrenal incidentalomas. Horm 2021 20, 1–10 (2021). https://doi.org/10.1007/S42000-021-00308-Z
M. Araujo-Castro, P. Valderrábano, H.F. Escobar-Morreale, F.A. Hanzu, G. Casals, Urine steroid profile as a new promising tool for the evaluation of adrenal tumors. Lit. Rev. Endocr. 72, 40–48 (2020). https://doi.org/10.1007/s12020-020-02544-6
M. Araujo-Castro, G. Casals G., F.A. Hanzu, E. Pascual-Corrales, A.M. García Cano, V.F. Lanza, et al. Characterisation of the urinary steroid profile of patients with nonfunctioning adrenal incidentalomas: A matched controlled cross-sectional study. Clin. Endocrinol. 2022. https://doi.org/10.1111/cen.14811
M. Araujo-Castro, M.A. Sampedro Núñez, M. Marazuela, Autonomous cortisol secretion in adrenal incidentalomas. Endocrine 2019;64. https://doi.org/10.1007/s12020-019-01888-y
C.H.L Shackleton. Steroid profiling: diagnosis of disorders affecting steroid synthesis and metabolism. In: M.L Gross, W Niessen, R.M Caprioli, (eds.) The Encyclopedia of Mass Spectrometry. 8 Elsevier: Amsterdam, 2006; 789–813
C.H.L. Shackleton, Mass spectrometry in the diagnosis of steroid-related disorders and in hypertension research. J. Steroid Biochem. Mol. Biol. 45, 127–140 (1993). https://doi.org/10.1016/0960-0760(93)90132-G
G. Casals, J. Marcos, O.J. Pozo, J. Alcaraz, M.J. Martínez de Osaba, W. Jiménez, Microwave-assisted derivatization: application to steroid profiling by gas chromatography/mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 960, 8–13 (2014). https://doi.org/10.1016/j.jchromb.2014.04.015
G. Casals, J. Marcos, Ó.J. Pozo, P. Aguilera, C. Herrero, J. To-Figueras, Gas chromatography-mass spectrometry profiling of steroids in urine of patients with acute intermittent porphyria. Clin. Biochem. 46, 819–824 (2013). https://doi.org/10.1016/j.clinbiochem.2013.03.001
A. Vega-Beyhart, J. Laguna-Moreno, D. Díaz-Catalán, L. Boswell, M. Mora, I. Halperin, et al. Ketoconazole- and metyrapone-induced reductions on urinary steroid metabolites alter the urinary free cortisol immunoassay reliability in cushing syndrome. Front. Endocrinol. 2022;13. https://doi.org/10.3389/FENDO.2022.833644
P.A. Harris, R. Taylor, R. Thielke, J. Payne, N. Gonzalez, J.G. Conde, Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009). https://doi.org/10.1016/j.jbi.2008.08.010
P.A. Harris, R. Taylor, B.L. Minor, V. Elliott, M. Fernandez, L. O’Neal, et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 2019;95. https://doi.org/10.1016/j.jbi.2019.103208.
J. Brossaud, D. Ducint, J.B. Corcuff, Urinary glucocorticoid metabolites: biomarkers to classify adrenal incidentalomas. Clin. Endocrinol. 84, 236–243 (2016). https://doi.org/10.1111/cen.12717
A. Kotłowska, E. Maliński, K. Sworczak, J. Kumirska, P. Stepnowski, The urinary steroid profile in patients diagnosed with adrenal incidentaloma. Clin. Biochem. 42, 448–454 (2009). https://doi.org/10.1016/j.clinbiochem.2008.12.027
M. Araujo-Castro, H.F. Escobar-Morreale, P. Valderrábano, P. Valderrabano, A proposal for nomenclature revision of non-functioning adrenal incidentalomas as adrenal lesions of Undetermined Secretion of Adrenal Steroids (ALUSAS). Endocr. Pract. 28, 918–920 (2022). https://doi.org/10.1016/J.EPRAC.2022.06.007
T.M.A. Kerkhofs, M.N. Kerstens, I.P. Kema, T.P. Willems, H.R. Haak, Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm. Cancer 6, 168–175 (2015). https://doi.org/10.1007/s12672-015-0224-3
A. Kotłowska, K. Sworczak, P. Stepnowski, Urine metabolomics analysis for adrenal incidentaloma activity detection and biomarker discovery. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 879, 359–363 (2011). https://doi.org/10.1016/j.jchromb.2010.12.021
A. Kotłowska, T. Puzyn, K. Sworczak, P. Stepnowski, P. Szefer, Metabolomic biomarkers in urine of cushing’s syndrome patients. Int J. Mol. Sci. 18, 1–15 (2017). https://doi.org/10.3390/ijms18020294
O. Chabre, The difficulties of pseudo-Cushing’s syndrome (or “non-neoplastic hypercortisolism”). Ann. Endocrinol. 79, 138–145 (2018). https://doi.org/10.1016/J.ANDO.2018.04.017
Y.J. Bae, J. Kratzsch, Corticosteroid-binding globulin: modulating mechanisms of bioavailability of cortisol and its clinical implications. Best. Pract. Res Clin. Endocrinol. Metab. 29, 761–772 (2015). https://doi.org/10.1016/J.BEEM.2015.09.001
A.W. MEIKLE, Dexamethasone suppression tests: usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clin. Endocrinol. 16, 401–408 (1982). https://doi.org/10.1111/j.1365-2265.1982.tb00733.x
W. Liu, D. Yuan, M. Han, J. Huang, Y. **e, Development and validation of a sensitive LC-MS/MS method for simultaneous quantification of thirteen steroid hormones in human serum and its application to the study of type 2 diabetes mellitus. J. Pharm. Biomed. Anal. 2021;199. https://doi.org/10.1016/J.JPBA.2021.114059
V.L. Wester, J.W. Koper, E.L.T. Van Den Akker, O.H. Franco, R. P. Stolk, E.F.C. Van Rossum, Glucocorticoid receptor haplotype and metabolic syndrome: the Lifelines cohort study. Eur. J. Endocrinol. 175, 645–651 (2016). https://doi.org/10.1530/EJE-16-0534
M.S. Cooper, E.H. Rabbitt, P.E. Goddard, W.A. Bartlett, M. Hewison, P.M. Stewart, Osteoblastic 11beta-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J. Bone Min. Res 17, 979–986 (2002). https://doi.org/10.1359/JBMR.2002.17.6.979
V. Morelli, C. Aresta, A. Gaudio, C. Eller-Vainicher, V.V. Zhukouskaya, D. Merlotti et al. Prediction of hypertension, diabetes and fractures in eucortisolemic women by measuring parameters of cortisol milieu. Endocrine 68, 411–419 (2020). https://doi.org/10.1007/S12020-020-02212-9
T. Deutschbein, G. Reimondo, G. Di Dalmazi, I. Bancos, J. Patrova, D.A. Vassiliadi, et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: an international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022. https://doi.org/10.1016/S2213-8587(22)00100-0
V. Bansal, N. Asmar, W.W.R. Selman, B.B.M. Arafah, N. El Asmar, W.W.R. Selman et al. Pitfalls in the diagnosis and management of Cushing’s syndrome. Neurosurg. Focus 38, 1–11 (2015). https://doi.org/10.3171/2014.11.FOCUS14704.Disclosure
V. Chortis, I. Bancos, T. Nijman, L.C. Gilligan, A.E. Taylor, C.L. Ronchi, et al. Urine steroid metabolomics as a novel tool for detection of recurrent adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2020;105. https://doi.org/10.1210/clinem/dgz141
I. Bancos, W. Arlt, Diagnosis of a malignant adrenal mass: the role of urinary steroid metabolite profiling. Curr. Opin. Endocrinol. Diabetes Obes. 24, 200–207 (2017). https://doi.org/10.1097/MED.0000000000000333
Funding
SENDIMAD: BECA SENDIMAD de Ayuda a la Investigación en Endocrinología, Nutrición y Diabetes 2019. IRYCIS: Convocatoria intramural de ayudas a proyectos de investigación de investigadores noveles, investigadores clínicos asociados y/o grupos emergentes del Hospital Universitario Ramón y Cajal 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures performed in the participants of the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All patients signed an informed consent to participate in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Araujo-Castro, M., Hanzu, F.A., Pascual-Corrales, E. et al. Is the 1mg-dexamethasone suppression test a precise marker of glucocorticoid excess and cardiometabolic risk in patients with adrenal incidentalomas?. Endocrine 82, 161–170 (2023). https://doi.org/10.1007/s12020-023-03429-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03429-0