Abstract
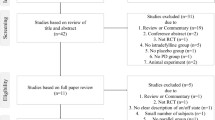
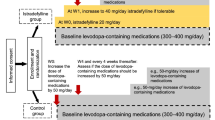
Adenosine A2A receptor antagonist istradefylline has been approved this year for manufacturing and marketing in Japan. We, therefore, did this meta-analysis to systematically assess the efficacy and safety of istradefylline as augmentation to levodopa in patients with Parkinson’s disease (PD). We systematically review the relative randomized controlled trials (RCTs) up to March 2014, which compared istradefylline to placebo for the short course of treatment for PD in adults. The primary outcome was daily OFF time and secondary outcome was UPDRS Part III score (on state). Data were obtained from seven RCTs, including 2205 patients. Compared to placebo on primary and secondary outcome, istradefylline group both showed significant reductions (WMD −0.60, p = 0.0001; WMD −1.07, p = 0.002). Subgroup analysis suggested that istradefylline 20, 40, and 60 mg/day in both group showed significant reductions on the two outcomes. Based on these results, Istradefylline could be an efficacy and safety augmentation drug added on to levodopa or other existing anti-Parkinsonian therapies. Limited by the number of studies, future large-scale studies are needed to verify these results, assess the long-term effect of istradefylline and the effect of istradefylline as monotherapy, and find the most effective dose of istradefylline.



Similar content being viewed by others
References
Fahn, S. (2003). Description of Parkinson’s disease as a clinical syndrome. Annals of the New York Academy of Sciences, 991, 1–14.
Barzilai, A., & Melamed, E. (2003). Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends in Molecular Medicine, 9(3), 126–132.
Dauer, W., & Przedborski, S. (2003). Parkinson’s disease: mechanisms and models. Neuron, 39(6), 889–909.
Dorsey, E. R., et al. (2007). Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology, 68(5), 384–386.
Bonuccelli, U. (2003). Comparing dopamine agonists in Parkinson’s disease. Current Opinion in Neurology, 16(Suppl 1), S13–S19.
Jenner, P. (2003). Dopamine agonists, receptor selectivity and dyskinesia induction in Parkinson’s disease. Current Opinion in Neurology, 16(Suppl 1), S3–S7.
Cieslak, M., Komoszynski, M., & Wojtczak, A. (2008). Adenosine A(2A) receptors in Parkinson’s disease treatment. Purinergic Signal, 4(4), 305–312.
Berlim, M. T., Van den Eynde, F., & Daskalakis, Z. J. (2013). A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychological Medicine, 43(11), 2245–2254.
Chen, J., et al. (2013). Left versus right repetitive transcranial magnetic stimulation in treating major depression: A meta-analysis of randomised controlled trials. Psychiatry Research, 210(3), 1260–1264.
Shiozaki, S., et al. (1999). Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hypokinesia caused by reserpine or MPTP. Psychopharmacology (Berl), 147(1), 90–95.
Wenning, G. K., et al. (2013). The natural history of multiple system atrophy: A prospective European cohort study. Lancet Neurology, 12(3), 264–274.
Hauser, R. A., et al. (2008). Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Movement Disorders, 23(15), 2177–2185.
LeWitt, P. A., et al. (2008). Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: A double-blind, randomized, multicenter clinical trial (6002-US-005). Annals of Neurology, 63(3), 295–302.
Stacy, M., et al. (2008). A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology, 70(23), 2233–2240.
Dungo, R., & Deeks, E. D. (2013). Istradefylline: First global approval. Drugs, 73(8), 875–882.
Hughes, A. J., et al. (1992). What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology, 42(6), 1142–1146.
Sacks, H. S., et al. (1987). Meta-analyses of randomized controlled trials. New England Journal of Medicine, 316(8), 450–455.
Riley, R. D., Higgins, J. P., & Deeks, J. J. (2011). Interpretation of random effects meta-analyses. BMJ, 342, d549.
Higgins, J. P., et al. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560.
Pourcher, E., et al. (2012). Istradefylline for Parkinson’s disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study. Parkinsonism and Related Disorders, 18(2), 178–184.
Mizuno, Y., Kondo, T., & G. Japanese Istradefylline Study. (2013). Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Movement Disorders, 28(8), 1138–1141.
Mizuno, Y., et al. (2010). Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: A randomized, controlled study. Movement Disorders, 25(10), 1437–1443.
Hauser, R. A., & Holford, N. H. (2002). Quantitative description of loss of clinical benefit following withdrawal of levodopa-carbidopa and bromocriptine in early Parkinson’s disease. Movement Disorders, 17(5), 961–968.
Kanda, T., et al. (2000). Combined use of the adenosine A(2A) antagonist KW-6002 with L-DOPA or with selective D1 or D2 dopamine agonists increases antiparkinsonian activity but not dyskinesia in MPTP-treated monkeys. Experimental Neurology, 162(2), 321–327.
Grondin, R., et al. (1999). Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology, 52(8), 1673–1677.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tao, Y., Liang, G. Efficacy of Adenosine A2A Receptor Antagonist Istradefylline as Augmentation for Parkinson’s Disease: A Meta-analysis of Randomized Controlled Trials. Cell Biochem Biophys 71, 57–62 (2015). https://doi.org/10.1007/s12013-014-0162-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0162-7