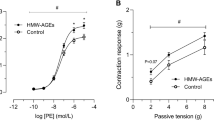
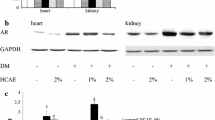
Abstract
The beneficial effect of curcumin (CU) on dietary AGEs (dAGEs) involves blocking the overexpression of proinflammatory cytokine genes in the heart and kidney tissues of experimental mice. The animals were divided into six groups (n = 6/group) and were fed a heat-exposed diet (dAGEs) with or without CU for 6 months. Their blood pressure (BP) was monitored by a computerized tail-cuff BP-monitoring system. The mRNA and protein expression levels of proinflammatory genes were analyzed by RT-PCR and western blot, respectively. A marked increase in BP (108 ± 12 mmHg vs 149 ± 15 mmHg) accompanied by a marked increase in the heart and kidney weight ratio was noted in the dAGE-fed mice. Furthermore, the plasma levels of proinflammatory molecules (C5a, ICAM-1, IL-6, MCP-1, IL-1β and TNF-α) were found to be elevated (3-fold) in dAGE-fed mice. mRNA expression analysis revealed a significant increase in the expression levels of inflammatory markers (Cox-2, iNOS, and NF-κB) (3-fold) in cardiac and renal tissues of dAGE-fed mice. Moreover, increased expression of RAGE and downregulation of AGER-1 (p < 0.001) were noticed in the heart and kidney tissues of dAGE-fed mice. Interestingly, the dAGE-induced proinflammatory genes and inflammatory responses were neutralized upon cotreatment with CU. The present study demonstrates that dietary supplementation with CU has the ability to neutralize dAGE-induced adverse effects and alleviate proinflammatory gene expression in the heart and kidney tissues of experimental mice.







Similar content being viewed by others
References
Vlassara, H., Cai, W., Tripp, E., Pyzik, R., Yee, K., Goldberg, L., et al. (2016). Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia, 59, 2181–2192.
Crisóstomo, J., Matafome, P., Santos-Silva, D., Rodrigues, L., Sena, C. M., et al. (2013). Methylglyoxal chronic administration promotes diabetes-like cardiac ischaemia disease in Wistar normal rats. Nutrition, Metabolism and Cardiovascular Diseases, 23, 1223–1230.
Abe, Y., Yagi, M., Uwaya, A., Isami, F., & Yonei, Y. (2016). Effect of iridoid (containing plants) on AGE formation and degradation. Glycative Stress Res, 3, 56–64.
Sveen, K. A., Dahl-Jørgensen, K., Stensaeth, K. H., Angel, K., Seljeflot, I., et al. (2015). Glucosepane and oxidative markers in skin collagen correlate with intima media thickness and arterial stiffness in long-term type 1 diabetes. Journal of Diabetes and its Complications, 29, 407–412.
Qais, F. A., Alam, M. M., Naseem, I., & Ahmad, I. (2016). Understanding the mechanism of non-enzymatic glycation inhibition by cinnamic acid: An in vitro interaction and molecular modelling study. RSC Advances, 6, 65322–65337.
Madsen-Bouterse, S. A., & Kowluru, R. A. (2008). Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Reviews in Endocrine and Metabolic Disorders, 9(4), 315–327.
Tobon-Velasco, J., Cuevas, E., & Torres-Ramos, M. (2014). Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 13, 1615–1626.
Vlassara, H., Cai, W., Goodman, S., Pyzik, R., Yong, A., Chen, X., Zhu, L., Neade, T., Beeri, M., Silverman, J. M., & Ferrucci, L. (2009). Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the anti -inflammatory AGE receptor-1. The Journal of Clinical Endocrinology & Metabolism, 94, 4483–4491.
Nowotny, K., Schröter, D., Schreiner, M., & Grune, T. (2018). Dietary advanced glycation end products and their relevance for human health. Ageing Research Reviews, 47, 55–66.
Goldberg, T., Cai, W., Peppa, M., Dardaine, V., Baliga, B. S., Uribarri, J., & Vlassara, H. (2004). Advanced glycoxidation end products in commonly consumed foods. Journal of the American Dietetic Association., 104, 1287–1291.
Newens, K. J., & Walton, J. (2016). A review of sugar consumption from nationally representative dietary surveys across the world. Journal of Human Nutrition and Dietetics, 29, 225–240.
Sharma, C., Kaur, A., Thind, S. S., Singh, B., & Raina, S. (2015). Advanced glycation end-products (AGEs): An emerging concern for processed food industries. Journal of Food Science and Technology, 52, 7561–7576.
Clarke, R. E., Dordevic, A. L., Tan, S. M., Ryan, L., & Coughlan, M. T. (2016). Dietary advanced glycation end products and risk factors for chronic disease: A systematic review of randomised controlled trials. Nutrients, 8, 125.
Delgado-Andrade, C., & Fogliano, V. (2018). Dietary advanced glycosylation end-products (dAGEs) and melanoidins formed through the Maillard reaction: Physiological consequences of their intake. Annual Review of Food Science and Technology, 9, 271–291.
Di Pino, A., Currenti, W., Urbano, F., Scicali, R., Piro, S., Purrello, F., & Rabuazzo, A. M. (2017). High intake of dietary advanced glycation end-products is associated with increased arterial stiffness and inflammation in subjects with type 2 diabetes. Nutrition, Metabolism and Cardiovascular Diseases, 27, 978–984.
**e, J., Méndez, J. D., Méndez-Valenzuela, V., & Aguilar-Hernández, M. M. (2013). Cellular signalling of the receptor for advanced glycation end products (RAGE). Cellular Signalling, 25, 2185–2197.
Bolton, W. K., Cattran, D. C., Williams, M. E., Adler, S. G., Appel, G. B., Cartwright, K., et al. (2004). Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. American Journal of Nephrology, 24, 32–40.
Kapakos, G., Youreva, V., & Srivastava, A. K. (2012). Cardiovascular protection by curcumin: Molecular aspects.
Prasad, S., Gupta, S. C., Tyagi, A. K., & Aggarwal, B. B. (2014). Curcumin, a component of golden spice: From bedside to bench and back. Biotechnology Advances., 32, 1053–1064.
Sandur, S. K., Ichikawa, H., Pandey, M. K., Kunnumakkara, A. B., Sung, B., Sethi, G., et al. (2007). Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radical Biology and Medicine, 43, 568–580.
Zong, H., Wang, F., Fan, Q. X., & Wang, L. X. (2012). Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Molecular Biology Reports., 39, 4803–4808.
Hu, T. Y., Liu, C. L., Chyau, C. C., & Hu, M. L. (2012). Trap** of methylglyoxal by curcumin in cell-free systems and in human umbilical vein endothelial cells. Journal of Agricultural and Food Chemistry, 60, 8190–8196.
Hu, T. Y., Liu, C. L., Chen, J. Y., & Hu, M. L. (2013). Curcumin ameliorates methylglyoxal-induced alterations of cellular morphology and hyperpermeability in human umbilical vein endothelial cells. Journal of Functional Foods, 5, 745–754.
Rajan, B. S., Manivasagam, S., Dhanusu, S., Chandrasekar, N., Krishna, K., Kalaiarasu, L. P., et al. (2018). Diet with high content of advanced glycation end products induces systemic inflammation and weight gain in experimental mice: Protective role of curcumin and gallic acid. Food and Chemical Toxicology, 114, 237–245.
Palombo, R., Gertler, A., & Saguy, I. (1984). A simplified method for determination of browning in dairy powders. Journal of Food Science, 49(6), 1609–1609.
Halatchev, I. G., Ellacott, K. L., Fan, W., & Cone, R. D. (2004). Peptide YY3–36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology, 145(6), 2585–2590.
Johnson, M. S., Thomson, S. C., & Speakman, J. R. (2001). Limits to sustained energy intake: I. Lactation in the laboratory mouse MUS MUSCULUS. Journal of Experimental Biology, 204, 1925–1935.
Ling, Q. L., Mohite, A. J., Murdoch, E., Akasaka, H., Li, Q. Y., So, S. P., et al. (2018). Creating a mouse model resistant to induced ischemic stroke and cardiovascular damage. Scientific Reports, 8(1), 1653.
Ushijima, T., Fujimoto, N., Matsuyama, S., Kan-o, M., Kiyonari, H., Shioi, G., ... Sumimoto, H. (2018). The actin-organizing formin protein Fhod3 is required for postnatal development and functional maintenance of the adult heart in mice. Journal of Biological Chemistry, 293(1), 148–162.
Frantz, S., Fraccarollo, D., Wagner, H., Behr, T. M., Jung, P., Angermann, C. E., Ertl, G., & Bauersachs, J. (2003). Sustained activation of nuclear factor kappa B and activator protein 1 in chronic heart failure. Cardiovascular Research., 57(3), 749–756.
Maiuolo, J., Maretta, A., Gliozzi, M., Musolino, V., Carresi, C., Bosco, F., ... Nucera, S. (2018). Ethanol-induced cardiomyocyte toxicity implicit autophagy and NFkB transcription factor. Pharmacological Research, 133, 141–150.
Joseph, D. R. (1908). The ratio between the heart-weight and body-weight in various animals. The Journal of Experimental Medicine, 10, 521.
Tighe, P. J., Ryder, R. R., Todd, I., & Fairclough, L. C. (2015). ELISA in the multiplex era: Potentials and pitfalls. PROTEOMICS–Clinical Applications , 9, 406–422.
Uribarri, J., Woodruff, S., Goodman, S., Cai, W., Chen, X., Pyzik, R., et al. (2010). Advanced glycation end products in foods and a practical guide to their reduction in the diet. Journal of the American Dietetic Association, 110, 911–916.
Palioura, E., Palimeri, S., Piperi, C., Sakellariou, S., Kandaraki, E., Sergentanis, T., et al. (2015). Impact of androgen and dietary advanced glycation end products on female rat liver. Cellular Physiology and Biochemistry, 37, 1134–1146.
Cai, W., Ramdas, M., Zhu, L., Chen, X., Striker, G. E., & Vlassara, H. (2012). Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proceedings of the National Academy of Sciences, 109, 15888–15893.
Fabre, N. T., Thieme, K., Silva, K. S., Catanozi, S., Cavaleiro, A. M., Pinto, D. A., Jr., et al. (2017). Hormetic modulation of hepatic insulin sensitivity by advanced glycation end products. Molecular and Cellular Endocrinology, 447, 116–124.
Um, M. Y., Hwang, K. H., Ahn, J., & Ha, T. Y. (2013). Curcumin attenuates diet-induced hepatic steatosis by activating AMPactivated protein kinase. Basic & Clinical Pharmacology & Toxicology, 113, 152–157.
Maithili Karpaga Selvi, N., Sridhar, M. G., Swaminathan, R. P., & Sripradha, R. (2015). Curcumin attenuates oxidative stress and activation of redox-sensitive kinases in high fructose-and high-fat-fed male Wistar rats. Scientia Pharmaceutica, 83, 159–175.
Di Pierro, F., Bressan, A., Ranaldi, D., Rapacioli, G., Giacomelli, L., & Bertuccioli, A. (2015). Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. European Review for Medical and Pharmacological Sciences, 19, 4195–4202.
Chainani-Wu, N. (2003). Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). The Journal of Alternative & Complementary Medicine., 9, 161–168.
Shehzad, A., Ha, T., Subhan, F., & Lee, Y. S. (2011). New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. European Journal of Nutrition., 50, 151–161.
Weisberg, S. P., Leibel, R., & Tortoriello, D. V. (2008). Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology, 149, 3549–3558.
Mirmiran, P., Yousefi, R., Mottaghi, A., & Azizi, F. (2018). Advanced glycation end products and risk of hypertension in Iranian adults: Tehran lipid and glucose study. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences, 23, 43.
Wang, X., Desai, K., Clausen, J. T., & Wu, L. (2004). Increased methylglyoxal and advanced glycation end products in kidney from spontaneously hypertensive rats. Kidney International, 66, 2315–2321.
Umadevi, S., Gopi, V., & Elangovan, V. (2014). Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chemico-Biological Interactions, 208, 28–36.
Yousuf, M. J., & Vellaichamy, E. (2015). Protective activity of gallic acid against glyoxal-induced renal fibrosis in experimental rats. Toxicology Reports, 2, 1246–1254.
Akazawa, N., Choi, Y., Miyaki, A., Tanabe, Y., Sugawara, J., Ajisaka, R., & Maeda, S. (2012). Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutrition Research, 32, 795–799.
Morimoto, T., Sunagawa, Y., Kawamura, T., Takaya, T., Wada, H., Nagasawa, A., Komeda, M., et al. (2008). The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. The Journal of Clinical Investigation., 118, 868–878.
Zhang, H., Liu, H., Chen, Y., & Zhang, Y. (2018). The curcumin-induced vasorelaxation in rat superior mesenteric arteries. Annals of Vascular Surgery, 48, 233–240.
Sajithlal, G. B., Chithra, P., & Chandrakasan, G. (1998). Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochemical Pharmacology, 56, 1607–1614.
Stirban, A., & Tschöpe, D. (2015). Vascular effects of dietary advanced glycation end products. International Journal of Endocrinology. https://doi.org/10.1155/2015/836498
Grunwald, S., Krause, R., Bruch, M., Henle, T., & Brandsch, M. (2006). Transepithelial flux of early and advanced glycation compounds across Caco-2 cell monolayers and their interaction with intestinal amino acid and peptide transport systems. British Journal of Nutrition, 95, 1221–1228.
Hellwig, M., Geissler, S., Matthes, R., Peto, A., Silow, C., Brandsch, M., & Henle, T. (2011). Transport of free and peptide-bound glycated amino acids: Synthesis, transepithelial flux at caco-2 cell monolayers, and interaction with apical membrane transport proteins. ChemBioChem, 12, 1270–1279.
Koschinsky, T., He, C. J., Mitsuhashi, T., Bucala, R., Liu, C., Buenting, C., ... Vlassara, H. (1997). Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proceedings of the National Academy of Sciences, 94, 6474–6479.
Roncero-Ramos, I., Delgado-Andrade, C., Tessier, F. J., Niquet-Léridon, C., Strauch, C., Monnier, V. M., et al. (2013). Metabolic transit of Nε-carboxymethyl-lysine after consumption of AGEs from bread crust. Food & Function, 4, 1032–1039.
Uribarri, J., Cai, W., Pyzik, R., Goodman, S., Chen, X., Zhu, L., ... Vlassara, H. (2014). Suppression of native defense mechanisms, SIRT1 and PPARγ, by dietary glycoxidants precedes disease in adult humans; relevance to lifestyle-engendered chronic diseases. Amino Acids, 46, 301–309.
Stirban, A., Negrean, M., Götting, C., Stratmann, B., Gawlowski, T., Mueller-Roesel, M., ... Tschoepe, D. (2008). Leptin decreases postprandially in people with type 2 diabetes, an effect reduced by the cooking method. Hormone and Metabolic Research, 40, 896–900.
Scheijen, J. L., Hanssen, N. M., van Greevenbroek, M. M., Van der Kallen, C. J., Feskens, E. J., Stehouwer, C. D., et al. (2018). Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clinical Nutrition, 37, 919–925.
Foerster, A., & Henle, T. (2003). Glycation in food and metabolic transit of dietary AGEs (advanced glycation end-products): Studies on the urinary excretion of pyrraline. Biochemical Society Transactions, 31, 1383–1385.
Yu, W., Wu, J., Cai, F., **ang, J., Zha, W., Fan, D., et al. (2012). Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS ONE, 7, e52013.
Seo, K. I., Choi, M. S., Jung, U. J., Kim, H. J., Yeo, J., Jeon, S. M., & Lee, M. K. (2008). Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Molecular Nutrition & Food Research., 52, 995–1004.
Derk, J., MacLean, M., Juranek, J., & Schmidt, A. M. (2018). The receptor for advanced glycation endproducts (RAGE) and mediation of inflammatory neurodegeneration. Journal of Alzheimer’s disease & Parkinsonism. https://doi.org/10.4172/2161-0460.1000421
Yan, S. F., Ramasamy, R., & Schmidt, A. M. (2008). Mechanisms of disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nature Reviews Endocrinology, 4, 285.
Yamagishi, S. I., Ueda, S., & Okuda, S. (2007). Food-derived advanced glycation end products (AGEs): A novel therapeutic target for various disorders. Current Pharmaceutical Design., 13, 2832–2836.
Soetikno, V., Sari, F. R., Veeraveedu, P. T., Thandavarayan, R. A., Harima, M., Sukumaran, V., et al. (2011). Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutrition & Metabolism, 8, 35.
Farhangkhoee, H., Khan, Z. A., Chen, S., & Chakrabarti, S. (2006). Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutrition & Metabolism., 3, 27.
Vlassara, H., Cai, W., Goodman, S., Pyzik, R., Yong, A., Chen, X., et al. (2009). Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the anti-inflammatory AGE receptor-1. The Journal of Clinical Endocrinology & Metabolism, 94, 4483–4491.
Lin, J., Tang, Y., Kang, Q., Feng, Y., & Chen, A. (2012). Curcumin inhibits gene expression of receptor for advanced glycation end products (RAGE) in hepatic stellate cells in vitro by elevating PPARγ activity and attenuating oxidative stress. British Journal of Pharmacology, 166, 2212–2227.
Manetti, A. C., Maiese, A., Paolo, M. D., De Matteis, A., La Russa, R., Turillazzi, E., ... Fineschi, V. (2021). MicroRNAs and sepsis-induced cardiac dysfunction: A systematic review. International Journal of Molecular Sciences, 22(1), 321.
Sanz, A. B., Sanchez-Niño, M. D., Ramos, A. M., Moreno, J. A., Santamaria, B., Ruiz-Ortega, M., ... Ortiz, A. (2010). NF-κB in renal inflammation. Journal of the American Society of Nephrology, 21(8), 1254–1262.
**ang, M., Wang, J., Zhang, Y., Ling, J., & Xu, X. (2012). Attenuation of aortic injury by ursolic acid through RAGE-Nox-NFκB pathway in streptozocin-induced diabetic rats. Archives of Pharmacal Research, 35, 877–886.
Acknowledgements
Dr. EV acknowledges the Department of Biotechnology (DBT), India, for financial support in the form of a major research project (D.O.BT/PR6259/FNS/20/587/2012). BS thanks the Department of Biotechnology (DBT) and the Indian Council of Medical Research (ICMR) for research fellowships. KK thanks the Indian Council of Medical Research (ICMR) for the senior research fellowship (SRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there are no competing financial interests for this work.
Ethical Approval
All applicable CPCSEA-approved guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Handling Editor: Mitzi C. Glover.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12012_2021_9697_MOESM1_ESM.tif
Supplementary Fig. 1 (A-C) shows the texture, browning index and N ε-CML levels in the basal and heat-exposed AIN-76 diets (72°C and 90°C). Values are expressed as the mean ± SEM (n = 3). **p < 0.05, AIN-76 basal diet vs AIN-76 heat-exposed diet 72°C, *** p<0.01, AIN-76 basal diet vs AIN-76 heat-exposed diet 90°C. Supplementary file1 (TIF 32267 KB)
12012_2021_9697_MOESM2_ESM.tif
Supplementary Fig. 2 (A-D) shows the mRNA expression levels of hypertrophic marker genes in the heart and kidney tissue of the control and experimental groups of mice. BD - basal diet, HED – heat-exposed diet, N ε-CML – N ε-CML mixed basal diet, HED + CU - HED diet cotreated with curcumin, N ε-CML + CU – N ε-CML mixed diet cotreated with curcumin and BD+CU – basal diet alone treated with curcumin. The data are represented as box and whisker plots; the box represents the 1st and 3rd quartiles, and the heavy bar represents the median value. a p < 0.001 and b p<0.01, BD-fed mice vs HED- and N ε-CML mixed basal diet-fed mice, respectively; a*p<0.05, HED vs HED + CU cotreated mice, b*p<0.05, N ε-CML vs N ε-CML +CU cotreated mice; ns - Nonsignificant changes in BD + CU-alone treated vs control mice. Supplementary file2 (TIF 22119 KB)
Rights and permissions
About this article
Cite this article
Sowndhar Rajan, B., Krishnan, K. & Vellaichamy, E. Diet-Derived Advanced Glycation End Products (dAGEs) Induce Proinflammatory Cytokine Expression in Cardiac and Renal Tissues of Experimental Mice: Protective Effect of Curcumin. Cardiovasc Toxicol 22, 35–51 (2022). https://doi.org/10.1007/s12012-021-09697-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09697-4