Abstract
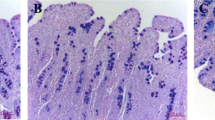
The current study was conducted to investigate the effect of zinc supplementation on the growth performance, intestinal morphology, and the transcription of the barrier function related genes in Pekin ducks. Seven-hundred and sixty-eight 1-day-old Pekin ducks were randomly assigned into six dietary treatments. Each treatment had eight replicates with 16 ducks per replicates. The ducks were fed either a corn-soybean meal basal diet or basal diets supplemented with 15, 30, 60, 120, and 240 mg zinc/kg from zinc sulfate. This experiment lasted for 5 weeks, and the jejunum sample were harvested at 14 and 35 days of age. Results have shown that diets supplemented with zinc significantly increased the duck body weight, average daily gain, and average daily feed intake in different period of experiment (P < 0.05); feed to gain ratio was decreased as the zinc level increased (P < 0.05). Zinc supplementation increased the villus height and decreased the crypt depth in jejunum of ducks (P < 0.05) at 14 and 35 days of age. The transcription of tight junction protein CLDN1, OCND, ZO-1, and ZO-3 in jejunum were increased (P < 0.05), and the messenger RNA (mRNA) levels of leak protein CLDN2 were decreased as the dietary zinc level increased (P < 0.05) at 14 and 35 days of age. The mRNA levels of chemical barrier-related genes MUC2 and TFF-2 in jejunum at 14 and 35 days of age were increased (P < 0.05) by zinc supplementation, and so did the transcription of immunological barrier-related genes lgA, pIgR, LYZ, and AvBD2 (P < 0.05). In conclusion, dietary zinc supplementation exhibited growth-promoting effect on Pekin duck, improved intestinal morphology, and enhanced the intestinal barrier integrity.



Similar content being viewed by others

References
Andreini C, Banci L, Bertini I et al (2006) Counting the zinc-proteins encoded in the human genome. J Proteome Res 5:196–201
Andreini C, Bertini I (2012) A bioinformatics view of zinc enzymes. J Inorg Biochem 111:150–156
Salim HM, Jo C, Lee BD (2008) Zinc in broiler feeding and nutrition. Avian Biol Res 1:5–18
Feng J, Ma WQ, Niu HH et al (2010) Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol Trace Elem Res 133:203–211
Liu ZH, Lu L, Li SF et al (2011) Effects of supplemental zinc source and level on growth performance, carcass traits, and meat quality of broilers. Poult Sci 90:1782–1790
Zhang B, Shao Y, Liu D et al (2012) Zinc prevents Salmonella enterica serovar typhimurium-induced loss of intestinal mucosal barrier function in broiler chickens. Avian Pathol 41:361–367
Wu LY, Fang YJ, Guo XY (2011) Dietary L-arginine supplementation beneficially regulates body fat deposition of meat-type ducks. Br Poult Sci 52:221–226
Cui H, ** P, Junliang D et al (2004) Pathology of lymphoid organs in chickens fed a diet deficient in zinc. Avian Pathol 33:519–524
Wight PA, Dewar WA (1976) The histopathology of zinc deficiency in ducks. Avian Pathol 120:183–191
Cui H, **g F, ** P (2003) Pathology of the thymus, spleen and bursa of Fabricius in zinc-deficient ducklings. Avian Pathol 32:259–264
Applegate T, Karcher D, Lilburn M (2005) Comparative development of the small intestine in the turkey poult and Pekin duckling. Poult Sci 84:426–431
Halpern MD, Denning PW (2015) The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 3:e1000707
Vereecke L, Beyaert R, Van Loo G (2011) Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol Med 17:584–593
Zhu C, Lv H, Chen Z et al (2016) Dietary zinc oxide modulates antioxidant capacity, small intestine development, and jejunal gene expression in weaned piglets. Biol Trace Elem Res 175:331–338
Han XY, Ma YF, Lv MY et al (2014) Chitosan-zinc chelate improves intestinal structure and mucosal function and decreases apoptosis in ileal mucosal epithelial cells in weaned pigs. Br J Nutr 111:1405–1411
Shao Y, Lei Z, Yuan J et al (2014) Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with Salmonella enterica serovar typhimurium. J Microbiol 52:1002–1011
Hu CH, Qian ZC, Song J et al (2013) Effects of zinc oxide-montmorillonite hybrid on growth performance, intestinal structure, and function of broiler chicken. Poult Sci 92:143–150
Zhang BK, Guo YM (2009) Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr 102:687
Wang X, Valenzano MC, Mercado JM et al (2013) Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig Dis Sci 58:77–87
Wang X, Valenzano MC, Mercado JM et al (2014) Zinc enhancement of LLC-PK(1) renal epithelial barrier function. Clin Nutr 33:280–286
Ranaldi G, Caprini V, Sambuy Y et al (2009) Intracellular zinc stores protect the intestinal epithelium from ochratoxin A toxicity. Toxicol in Vitro 23:1516–1521
Liberato SC, Singh G, Mulholland K (2015) Zinc supplementation in young children: a review of the literature focusing on diarrhoea prevention and treatment. Clin Nutr 34:181–188
Zeng QF, Yang GL, Liu GN et al (2014) Effects of dietary gossypol concentration on growth performance, blood profiles, and hepatic histopathology in meat ducks. Poult Sci 93:2000–2009
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408
Wight PA (1977) The ultrastructure of the interdigital web in experimental zinc deficiency of ducks. Avian Pathol 6:111–124
Roberson RH, Schaible PJ (1960) The tolerance of growing chicks for high levels of different forms of zinc. Poult Sci 39:893–896
Southon S, Livesey G, Gee JM et al (1985) Intestinal cellular proliferation and protein synthesis in zinc-deficient rats. Br J Nutr 53:595
Park JH, Grandjean CJ (1986) Effects of isolated zinc deficiency on the composition of skeletal muscle, liver and bone during growth in rats. J Nutr 116:610–617
Macdonald RS, Hambidge M, Cousins RJ et al (2000) The role of zinc in growth and cell proliferation. J Nutr 130:1500S–1508S
Laron Z (2001) Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol 54:311–316
Mcnall AD, Etherton TD, Fosmire GJ (1995) The impaired growth induced by zinc deficiency in rats is associated with decreased expression of the hepatic insulin-like growth factor I and growth hormone receptor genes. J Nutr 125:874–879
Ninh NX, Thissen JP, Maiter D et al (1995) Reduced liver insulin-like growth factor-I gene expression in young zinc-deprived rats is associated with a decrease in liver growth hormone (GH) receptors and serum GH-binding protein. J Endocrinol 144:449–456
Xu ZR, Hu CH, **a MS et al (2003) Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci 82:1030–1036
Ma W, Niu H, Feng J et al (2010) Effects of zinc glycine chelate on oxidative stress, contents of trace elements, and intestinal morphology in broilers. Biol Trace Elem Res 142:546–556
Lawson MJ, Butler RN, Goland GJ et al (1988) Zinc deficiency is associated with suppression of colonocyte proliferation in the distal large bowel of rats. Biol Trace Elem Res 18:115–121
Duff M, Ettarh R (2002) Crypt cell production rate in the small intestine of the zinc-supplemented mouse. Cells Tissues Organs 172:21
Yegani M, Korver DR (2008) Factors affecting intestinal health in poultry. Poult Sci 87:2052–2063
Anderson RC, Dalziel JE, Gopal PK et al (2012) The role of intestinal barrier function in early life in the development of colitis. INTECH:67–74
Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153
Suzuki T (2013) Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 70:631–659
Hu CH, Song ZH, **ao K et al (2014) Zinc oxide influences intestinal integrity, the expressions of genes associated with inflammation and TLR4-myeloid differentiation factor 88 signaling pathways in weanling pigs. Innate Immun 20:478–486
Pearce SC, Sanz Fernandez MV, Torrison J et al (2015) Dietary organic zinc attenuates heat stress-induced changes in pig intestinal integrity and metabolism. J Anim Sci 93:4702
Wada M, Tamura A, Takahashi N et al (2013) Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 144:369–380
Rosenthal R, Milatz S, Krug SM et al (2010) Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 123:1913
Luettig J, Rosenthal R, Barmeyer C et al (2015) Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 3:e977176
Mcguckin MA, Lindén SK, Sutton P et al (2011) Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9:265–278
Johansson ME, Hansson GC (2016) Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 16:639–649
Bobíková K, Levkut M, Husáková E et al (2016) Effect of glycine-zinc complex on mucin and IgA expression, secretory IgA concentration and lengths of intestinal villi in chickens. J Comp Pathol 154:81
Jiang Z, Lossie AC, Applegate TJ (2011) Evolution of trefoil factor(s): genetic and spatio-temporal expression of trefoil factor 2 in the chicken (Gallus gallus domesticus). PLoS One 6:e22691
Tran CP, Cook GA, Yeomans ND et al (1999) Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut 44:636–642
Ando K, Fujiya M, Konishi H et al (2015) Heterogeneous nuclear ribonucleoprotein A1 improves the intestinal injury by regulating apoptosis through trefoil factor 2 in mice with anti-CD3-induced enteritis. Inflamm Bowel Dis 21:1541–1552
Pabst O (2012) New concepts in the generation and functions of IgA. Nat Rev Immunol 12:821–832
Spencer J, Klavinskis LS, Fraser LD (2012) The human intestinal IgA response; burning questions. Front Immunol 3:108
Shimada S, Kawaguchi-Miyashita M, Kushiro A et al (1999) Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol 163:5367–5373
Bevins CL, Salzman NH (2011) Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9:356–368
Sawada M, Takahashi K, Sawada S et al (1991) Selective killing of Paneth cells by intravenous administration of dithizone in rats. Int J Clin Exp Pathol 72:407
Geiser J, Venken K, De Lisle RC et al (2012) A mouse model of Acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet 8:e1002766
Liu P, Pieper R, Tedin L et al (2014) Effect of dietary zinc oxide on jejunal morphological and immunological characteristics in weaned piglets. J Anim Sci 92:5009–5018
Mao X, Qi S, Yu B et al (2013) Zn(2+) and L-isoleucine induce the expressions of porcine beta-defensins in IPEC-J2 cells. Mol Biol Rep 40:1547–1552
Acknowledgements
The present study was financially supported by the Science and Technology Support Program of Sichuan Province (Grant No. 2013NZ0054), and the research funding was also provided by Sichuan Longda Animal Husbandry Science and Technology Co., Ltd. (No. 009H2200).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, M., Zhao, H., Liu, G. et al. Effect of Zinc Supplementation on Growth Performance, Intestinal Development, and Intestinal Barrier-Related Gene Expression in Pekin Ducks. Biol Trace Elem Res 183, 351–360 (2018). https://doi.org/10.1007/s12011-017-1143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1143-7