Abstract
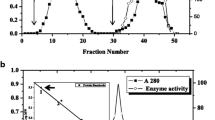
The highest β-mannanase activity was produced by Penicillium occitanis Pol6 on flour of carob seed, whereas starch-containing medium gave lower enzymes titles. The low molecular weight enzyme was purified to homogeneity by ammonium sulfate precipitation, gel filtration, and ion-exchange chromatography procedures. The purified β-mannanase (ManIII) has been identified as a glycoprotein (carbohydrate content 5%) with an apparent molecular mass of 18 kDa. It was active at 40 °C and pH 4.0. It was stable for 30 min at 70 °C and has a broad pH stability (2.0–12.0). ManIII showed K m, V max, and K cat values of 17.94 mg/ml, 93.52 U/mg, and 28.13 s−1 with locust bean gum as substrate, respectively. It was inhibited by mannose with a K I of 0.610−3 mg/ml. ManIII was activated by CuSO4 and CaCl2 (2.5 mM). However, in presence of 2.5 mM Co2+, its activity dropped to 60% of the initial activity. Both N-terminal and internal amino acid sequences of ManIII presented no homology with mannanases of glycosides hydrolases. During incubation with locust bean gum and Ivory nut mannan, the enzyme released mainly mannotetraose, mannotriose, and mannobiose.








Similar content being viewed by others
References
Timell, T. E. (1967). Wood Science and Technology, 1, 45–70. doi:10.1007/BF00592255.
Filho, E. X. F. (1998). Recent Research Developments in Microbiology, 2, 165–176.
Singh, S., Madlala, A. M., & Prior, B. A. (2003). FEMS Microbiology Reviews, 27, 3–16. doi:10.1016/S0168-6445(03)00018-4.
Xu, B., Hägglund, P., Stalbrand, H., & Janson, J. C. (2002). Journal of Biotechnology, 92, 267–277. doi:10.1016/S0168-1656(01)00367-4.
Duffaud, G. D., Mc cutchen, C. M., Leduc, P., Parker, K. N., & Kelly, R. M. (1997). Applied and Environmental Microbiology, 63, 169–177.
Gübitz, G. M., Hayn, M., Sommerauer, M., & Steiner, W. (1996). Bioresource Technology, 58, 127–135. doi:10.1016/S0960-8524(96)00093-4.
Stalbrand, H., Suka-aho, M., Tenkanen, M., & Viikari, L. (1993). Journal of Biotechnology, 29, 229–242. doi:10.1016/0168-1656(93)90055-R.
Civas, A., Eberhard, R., Le Dizet, P., & Petek, F. (1984). Biochemical Journal, 219, 857–863.
Yamazaki, N., Sinner, M., & Dietrichs, H. H. (1976). Holzforschung, 30, 101–109.
Ooi, T., & Kikuchi, D. (1995). World Journal of Microbiology & Biotechnology, 11, 310–314. doi:10.1007/BF00367106.
Nicolas, P., Raetz, E., Reymond, S., & Sauvegeat, J. L. (1995). EP0676145, A1.
Dekker, R. F. H. (1979). In J. M. V. Blanshard & J. R. Mitchell (Eds.), Polysaccharides in food (pp. 93–108). London: Butterworth.
Christgau, S., Andersen, L. N., Kauppinen, S., Heldt-Hansen, H. P., & Dalboege, H. (1994a). Patent Novo-Nordisk, 9425576.
Akino, T., Nakamura, N., & Horiksoshi, K. (1998). Agricultural and Biological Chemistry, 52, 773–779.
Oda, Y., Komaki, T., & Tonomura, K. (1993). Journal of Fermentation and Bioengineering, 76, 14–18. doi:10.1016/0922-338X(93)90045-A.
Viikari, L., Kantelinen, A., Sundquist, J., & Linko, M. (1994). FEMS Microbiology Reviews, 13, 335–350. doi:10.1111/j.1574-6976.1994.tb00053.x.
Cuevas, W. A., Kantelinen, A., Tanner, P., Bodie, B., & Leskinen, S. (1996). In E. Srebtonik & K. Messner (Eds.), Biotechnology in the pulp and paper industry (pp. 123–126). Vienna: Facultas-Universităts.
Gubitz, G. M., Haltrich, D., Latal, B., & Steiner, W. (1997). Applied Microbiology and Biotechnology, 47, 658–662. doi:10.1007/s002530050991.
Henrissat, B., & Bairoch, A. (1993). The Biochemical Journal, 293, 781–788.
Koshland, P. J. (1953). Biological Reviews of the Cambridge Philosophical Society, 28, 416–436. doi:10.1111/j.1469-185X.1953.tb01386.x.
Gibbs, M. D., Saul, D. J., Luthi, E., & Bergquist, P. L. (1992). Applied and Environmental Microbiology, 58, 3864–3867.
Tunnicliffe, R. B., Bolam, D. N., Pell, G., Gilbert, H. J., & Williamson, M. P. (2005). Journal of Molecular Biology, 347, 287–296. doi:10.1016/j.jmb.2005.01.038.
Park, G. G., Kusakabe, I., Komatsu, Y., Kobayashi, H., Yasui, T., & Murakami, K. (1987). Agricultural and Biological Chemistry, 51, 2709–2716.
Ademark, P., Varga, A., Medve, J., Harjunpaa, V., Drakenberg, T., Tjerneld, F., et al. (1998). Journal of Biotechnology, 63, 199–210. doi:10.1016/S0168-1656(98)00086-8.
Arisan-Atac, I., Hodits, R., Kristufek, D., & Kubicek, C. P. (1993). Applied Microbiology and Biotechnology, 39, 58–62.
Ferreira, H. M., & Filho, E. X. F. (2004). Carbohydrate Polymers, 57, 23–29. doi:10.1016/j.carbpol.2004.02.010.
Gübitz, G. M., Hayn, M., Urbanz, G., & Steiner, W. (1996). Journal of Biotechnology, 45, 165–172. doi:10.1016/0168-1656(95)00158-1.
Hadj Taieb, N., Ellouz, S., Kammoun, A., & Ellouz, R. (1992). Applied Microbiology and Biotechnology, 37, 197–201. doi:10.1007/BF00178170.
Ellouz Chaabouni, S., Belguith, H., Hsairi, I., Mkad, M., & Ellouz, R. (1995). Applied Microbiology and Biotechnology, 43, 267–269. doi:10.1007/BF00172822.
Limem, F., Ellouz, S., Ghriri, R., & Marzouki, N. (1995). Enzyme and Microbial Technology, 17, 340–346. doi:10.1016/0141-0229(94)00033-6.
Bhiri, F., Ellouz Chaabouni, S., Limam, F., Ghriri, R., & Marzouki, N. (2008). Applied Biochemistry and Biotechnology, 149, 169–182. doi:10.1007/s12010-008-8146-y.
Ellouz Chaabouni, S., Limam, F., Mechichi, T., & Marzouki, N. (2005). Applied Biochemistry and Biotechnology, 125, 99–112. doi:10.1385/ABAB:125:2:099.
Hägglund, P., Eriksson, T., Collén, A., Nerinckx, W., Claeyssens, M., & Stålbrand, H. (2003). Journal of Biotechnology, 101, 37–48. doi:10.1016/S0168-1656(02)00290-0.
Jain, S., Parriche, M., Durand, H., & Tiraby, G. (1990). Enzyme and Microbial Technology, 12, 691–696. doi:10.1016/0141-0229(90)90009-F.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428. doi:10.1021/ac60147a030.
Rättö, M., & Poutanen, K. (1988). Biotechnology Letters, 10, 661–664. doi:10.1007/BF01024721.
Bradford, M. (1976). Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3.
Spiro, R. (1966). Methods in Enzymology, 256, 3–26. doi:10.1016/0076-6879(66)08005-4.
Laemmli, U. K. (1970). Nature, 227, 680–685. doi:10.1038/227680a0.
Hewick, R. M., Hunkapiller, M. W., Hood, L. E., & Dreyer, W. J. (1981). The Journal of Biological Chemistry, 256, 7990–7997.
Macaron, R., Acebal, C., Castillon, M. P., & Claeyssens, M. (1996). Biotechnology Letters, 18, 599–602. doi:10.1007/BF00140210.
Sachslehner, A., Nidetzky, B., Kulbe, K. D., & Haltrich, D. (1998). Applied and Environmental Microbiology, 64, 594–600.
Gubitz, G. M., & Steiner, W. (1995). ACS Symposium Series. American Chemical Society, 618, 319–331.
Puchart, V., Vrsanska, M., Svoboda, P., Pohl, J., Ogel, Z., & Biely, P. (2004). Biochimica et Biophysica Acta, 1674, 239–250.
Torrie, J. P., Senior, D. J., & Saddler, J. N. (1990). Applied Microbiology and Biotechnology, 34, 303–307. doi:10.1007/BF00170047.
Sachslehner, A., & Haltrich, D. (1999). FEMS Microbiology Letters, 177, 47–55.
Setati, M. E., Ademark, P., van Zyl, W. H., Hahn-hagerdal, B., & Stalbrand, H. (2001). Protein Expression and Purification, 21, 105–114. doi:10.1006/prep.2000.1371.
Zhengaiang, J., Yun, W., Daoyi, L., Lite, L., ****, C., & Isao, K. (2006). Carbohydrate Polymers, 66, 88–96. doi:10.1016/j.carbpol.2006.02.030.
Kusakabe, I., Park, G. G., Kumita, N., Yasui, T., & Murakami, K. (1988). Agricultural and Biological Chemistry, 52, 519–524.
Kobayashi, Y., Echigen, R., Mada, M., & Mutai, M. (1987). In T. Mitsuoka (Ed.), Intestinal flora and food factors (pp. 79–87). Tokyo: Gakkai Shuppan Centre.
Acknowledgments
This research was financially supported in part by “Ministére de la recheche scientifique, de la Technologie et du dévelopement de compétences”. We express our gratitude to Prof. G. Tiraby and Dr. H. Durand (Cayla Company, France) for kindly supplying the P. occitanis (Pol 6) strain used in this work. We are also grateful to Prof. Radhouane Ellouz, Prof. Hamadi Attia, Prof. Youssef Gargouri, Prof. Mongi Feki, and Prof. Jalel Bouzid, ENIS, Sfax, Tunisia, for their stimulating and beneficial discussion. We extend our gratitude to Prof. Hafed Mejdoub (FSS, Sfax, Tunisia) for the determination of the N-terminal sequence and to Mr Jamil Jaoua, Head of the English Unit at the Sfax Faculty of Science, for having proofread this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blibech, M., Ghorbel, R.E., Fakhfakh, I. et al. Purification and Characterization of a Low Molecular Weight of β-Mannanase from Penicillium occitanis Pol6. Appl Biochem Biotechnol 160, 1227–1240 (2010). https://doi.org/10.1007/s12010-009-8630-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8630-z