Abstract
In this paper, we focus upon the use of anabolic skeletal therapy for the treatment of postmenopausal and other forms of osteoporosis. The only anabolic skeletal agent currently available is a recombinant bioactive fragment of parathyroid hormone, PTH(1-34), known as teriparatide. The full length molecule, human PTH(1-84) is being investigated at this time as are other PTH molecules. Teriparatide improves bone quality by actions on bone turnover, bone density, bone size, and microarchitecture. In postmenopausal women with osteoporosis, teriparatide reduces the incidence for vertebral and nonvertebral fractures. In individuals who have been treated previously with an antiresorptive agent, the subsequent actions of teriparatide on bone density are delayed transiently if bone turnover is markedly suppressed. Combination therapy with teriparatide or PTH(1-84) and an antiresorptive does not appear, at this time, to offer advantages over the use of PTH or an antiresorptive alone. To maintain the gains in bone density with PTH, it is important to follow its use with an antiresorptive agent.
Similar content being viewed by others
References and Recommended Reading
Hauselmann HJ, Rizzoli R: A comprehensive review of treatments for postmenopausal osteoporosis. Osteoporos Int 2003, 14:2–12.
Rosen CJ: Postmenopausal osteoporosis. N Engl J Med 2005, 353:595–603.
Dempster DW, Cosman F, Kurland ES, et al.: Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 2001, 16:1846–1853.
Hodsman AB, Bauer DC, Dempster DW, et al.: Parathyroid hormone and teriparatide for the treatment of osteoporosis; a review of the evidence and suggested guidelines for its use. Endocr Rev 2005, 26:688–703.
Lindsay R, Silverman SL, Cooper C, et al.: Risk of new vertebral fracture in the year following a fracture. JAMA 2001, 285:320–323.
Neer RM, Arnaud CD, Zanchetta JR, et al.: Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001, 344:1434–1441. This pivotal clinical trial establishes the efficacy of teriparatide in reducing vertebral and nonvertebral fracture incidence in postmenopausal women with osteoporosis.
Body JJ, Gaich GA, Scheele WH, et al.: A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2002, 87:4528–4535.
Prince R, Sipos A, Hossain A, et al.: Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 2005, 20:1507–1513.
Gallagher JC, Genant HK, Crans GG, et al.: Teriparatide reduces the fracture risk associated with increasing number and severity of osteoporotic fractures. J Clin Endocrinol Metab 2005, 90:1583–1587.
Marcus R, Wang O, Satterwhite J, Mitlak B: The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res 2003, 18:18–23.
Kurland ES, Cosman F, McMahon DJ, et al.: Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 2000, 85:3069–3076. This publication represents the first clinical trial of teriparatide in men with osteoporosis demonstrating its actions to increase bone density and providing an experimental basis to the concept of the anabolic window.
Orwoll ES, Scheele WH, Paul S, et al.: The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 2003, 18:9–17. The largest clinical trial to date on the use of teriparatide in men with osteoporosis. With a larger number of subjects than employed in the report of Kurland et al. [11•], this paper helps to document the efficacy of teriparatide in men with idiopathic osteoporosis or in those with hypogonadism.
Kaufman JM, Orwoll E, Goemaere S, et al.: Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int 2005, 16:510–516.
Rubin MR, Bilezikian JP: Parathyroid hormone as an anabolic skeletal therapy. Drugs 2005, 65:2481–2498.
Chen P, Satterwhite JH, Licata AA, et al.: Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res 2005, 20:962–970.
Dobnig H, Sipos A, Jiang Y, et al.: Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab 2005, 90:3970–3977.
Hodsman AB, Hanley DA, Ettinger MP, et al.: Efficacy and safety of human parathyroid hormone-(1-84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab 2003, 88:5212–5220.
Greenspan SL, Bone HG, Marriott TB, et al.: Preventing the first vertebral fracture in postmenopausal women with low bone mass using PTH(1-84): results from the TOP Study. J Bone Miner Res 2005, 20(Suppl1):S56.
Jiang Y, Zhao JJ, Mitlak BH, et al.: Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 2003, 18:1932–1941.
Recker R, Bare S, Miller M, et al.: Treatment of osteoporotic women with parathyroid hormone 1–84 for 18 months improves cancellous bone formation and structure; a bone biopsy study. J Bone Miner Res 2004, 19(Supp1):S97.
Burr DB, Hirano T, Turner CH, et al.: Intermittently administered human parathyroid hormone(1-34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res 2001, 16:157–165.
Zanchetta JR, Bogado CE, Ferretti JL, et al.: Effects of teriparatide [recombinant human parathyroid hormone (1-34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res 2003, 18:539–543.
Hodsman AB, Kisiel M, Adachi JD, et al.: Histomorphometric evidence for increased bone turnover without change in cortical thickness or porosity after 2 years of cyclical hPTH(1-34) therapy in women with severe osteoporosis. Bone 2000, 27:311–318.
Mashiba T, Burr DB, Turner CH, et al.: Effects of human parathyroid hormone (1-34), LY333334, on bone mass, remodeling, and mechanical properties of cortical bone during the first remodeling cycle in rabbits. Bone 2001, 28:538–547.
Lindsay R, Nieves J, Formica C, et al.: Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997, 350:550–555.
Cosman F, Nieves J, Woelfert L, et al.: Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res 2001, 16:925–931.
Roe E, Sanchez S, del Puerto G, et al.: trogen produce dramatic bone density increases in postmenopausal osteoporosis-results from a placebo-controlled randomized trial. J Bone Miner Res 1999, 14(Suppl1):S137.
Ettinger B, San Martin J, Crans G, Pavo I: Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004, 19:745–751. A widely quoted article addressing an important issue, namely whether previous antiresorptive therapy affects the subsequent densitometric response to teriparatide. This paper led to the concept that marked suppression of bone turnover by antiresorptive therapy can lead to a delay in the densitometric response to teriparatide.
Cosman F, Nieves J, Zion M, et al.: Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 2005, 353:566–575. In this paper, the authors present an interesting new way to administer teriparatide. They compared the effects of a cyclical 3-month regimen with continuous teriparatide therapy over a 15 month period. In addition to showing that bone density improves to the same extent with either approach, they provide intriguing new possibility that this approach expands the anabolic window.
Rubin MR, Bilezikian JP: Clinical review 151: the role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a re-examination of the evidence. J Clin Endocrinol Metab 2002, 87:4033–4041.
Lane NE, Sanchez S, Modin GW, et al.: Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest 1998, 102:1627–1633.
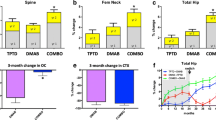
Black DM, Greenspan SL, Ensrud KE, et al.: The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003, 349:1207–1215. A seminal paper showing that combination therapy with PTH and alendronate does not provide for greater gains in bone density than PTH alone.
Finkelstein JS, Hayes A, Hunzelman JL, et al.: The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 2003, 349:1216–1226. Similar to the results of Black et al. [32••], this paper shows similar results when a group of men with osteoporosis were studied with combination antiresorptive and anabolic therapy.
Deal C, Omizo M, Schwartz EN, et al.: Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis; results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res, 2004 19(Supp1):1169.
Lindsay R, Scheele WH, Neer R, et al.: Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopasual women with osteoporosis. Arch Intern Med 2004, 164:2024–2030.
Rittmaster RS, Bolognese M, Ettinger MP, et al.: Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate [see comments]. J Clin Endocrinol Metab 2000, 85:2129–2134.
Lane NE, Sanchez S, Modin GW, et al.: Bone mass continues to increase at the hip after parathyroid hormone treatment is discontinued in glucocorticoid-induced osteoporosis: results of a randomized controlled clinical trial. J Bone Miner Res 2000, 15:944–951.
Kurland ES, Heller SL, Diamond B, et al.: The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone(1-34)]. Osteoporos Int 2004, 15:992–997.
Black DM, Bilezikian JP, Rosen C, et al.: The effect of one year of alendronate following one year of PTH1-84: second year results from the PTH and alendronate (PaTH) trial. N Engl J Med 2005, 353:555–566. This second PaTH paper provides data in a rigorously controlled trial that argues for the importance of antiresorptive therapy after anabolic skeletal therapy. The results show that when PTH is not followed up by alendronate, major losses in bone density, as measured by DXA or by QCT, are experienced. When alendronate is used after PTH, the gains in bone density are maintained or further enhanced.
Misof BM, Roschger P, Cosman F, et al.: Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab 2003, 88:1150–1156.
Dempster DW, Parisien M, Silverberg SJ, et al.: On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 1999, 84:1562–1566.
Vahle JL, Long GG, Sandusky G, et al.: Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol 2004, 32:426–438.
Wilker C, Jolette J, Smith S, et al.: No observable carcinogenic effect dose level identified in Fischer 344 rats following daily treatment with PTH(1-84) for 2 years: role of the C-terminal PTH receptor? J Bone Miner Res 2004, 19(Supp1):SA435.
Tashjian AH, Chabner BA: Commentary on clinical safety of recombinant human parathyroid hormone 1–34 in the treatment of osteoporosis in men and postmenopausal women. J Bone Miner Res 2002, 17:1151–1161.
Betancourt M, Wirfel KL, Raymond AK, et al.: Vassilopoulousellin, osteosarcoma of bone in apatient with primary hyperparathyroidism: a case report. J Bone Miner Res 2003, 18:163–166.
Wiig JN, Bakken TS: Hyperparathyroidism with multiple malignant tumours of bone with giant-cells. A case report. Acta Chir Scand 1971, 137:391–393.
Smith J, Huvos AG, Chapman M, et al.: Hyperparathyroidism associated with sarcoma of bone. Skeletal Radiol 1997, 26:107–112.
Palmer M, Adami HO, Krusemo UB, Ljunghall S: Increased risk of malignant diseases after surgery for primary hyperparathyroidism. A nationwide cohort study. Am J Epidemiol 1988, 127:1031–1040.
Jiminez C, Yang Y, Kim HW, et al.: Primary hyperparathyroidism and osteosarcoma: examination of a large cohort identifies three cases of fibroblastic osteosarcoma. J Bone Miner Res 2005, 20:1562–1568.
Gopalakrishnan V, Hwang S, Loughre H, et al.: Administration of ThPTH to humans using Macroflux transdermal technology reults in the rapid delivery of biologically active PTH. J Bone Miner Res 2004, 19(Supp1):M484.
Leone-Bay A, Sato M, Paton D, et al.: Oral delivery of biologically active parathyroid hormone. Pharm Res 2001, 18:964–970.
Fraher LJ, Avram R, Watson PH, et al.: Comparison of the biochemical responses to human parathyroid hormone-(1–31)NH2 and hPTH-(1-34) in healthy humans. J Clin Endocrinol Metab 1999, 84:2739–2743.
Horwitz MJ, Tedesco MB, Gundberg C, et al.: Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 2003, 88:569–575.
Black DM, Rosen CJ: Parsimony with PTH: Is a single weekly injection of PTH superior to a larger cumulative dose given daily? J Bone Miner Res 2002, 17(Suppl1):SA367.
Heaney RP, Recker RR: Combination and sequential therapy for osteoporosis. N Engl J Med 2005, 353:624–625.
Gowen M, Stroup GB, Dodds RA, et al.: Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest 2000, 105:1595–1604.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bilezikian, J.P., Rubin, M.R. Combination/sequential therapies for anabolic and antiresorptive skeletal agents for osteoporosis. Curr Osteoporos Rep 4, 5–13 (2006). https://doi.org/10.1007/s11914-006-0009-2
Issue Date:
DOI: https://doi.org/10.1007/s11914-006-0009-2