Abstract
Purpose of Review
This review will examine the taxonomy of PCa subclasses across disease states, explore the relationship among specific alterations, and highlight current clinical relevance.
Recent Findings
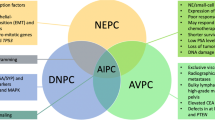
Prostate cancer (PCa) is driven by multiple genomic alterations, with distinct patterns and clinical implications. Alterations occurring early in the timeline of the disease define core subtypes of localized, treatment-naive PCa. With time, an increase in number and severity of genomic alterations adds molecular complexity and is associated with progression to metastasis. These later events are not random and are influenced by the underlying subclasses.
Summary
All the subclasses of localized disease initially respond to androgen deprivation therapy (ADT), but with progression to castrate-resistant PCa (CRPC), mechanisms of resistance against ADT shift the molecular landscape. In CRPC, resistance mechanisms largely define the biology and sub-classification of these cancers, while clinical relevance and opportunities for precision therapy are still being defined.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance ••Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46(10):1103–9. https://doi.org/10.1038/ng.3094.
Brawer MK. Prostatic intraepithelial neoplasia: an overview. Rev Urol. 2005;7(Suppl 3):S11–8.
Damber JE, Aus G. Prostate cancer. Lancet. 2008;371(9625):1710–21. https://doi.org/10.1016/S0140-6736(08)60729-1.
Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90(10):766–71.
Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180–92. https://doi.org/10.1111/j.1742-1241.2011.02799.x.
Schweizer MT, Yu EY. Persistent androgen receptor addiction in castration-resistant prostate cancer. J Hematol Oncol. 2015;8:128. https://doi.org/10.1186/s13045-015-0225-2.
Koryakina Y, Ta HQ, Gioeli D. Androgen receptor phosphorylation: biological context and functional consequences. Endocr Relat Cancer. 2014;21(4):T131–45. https://doi.org/10.1530/ERC-13-0472.
•• Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–28. https://doi.org/10.1016/j.cell.2015.05.001. This study provided clinical molecular analysis of advanced prostate cancers and reported the molecular alterations that add heterogeneity observed in patients with CRPC. It also provided potential clinical utility of patients' molecular profiles as predictive markers for the precision therapies.
Salonen AJ, Viitanen J, Lundstedt S, Ala-Opas M, Taari K, Tammela TL, et al. Finnish multicenter study comparing intermittent to continuous androgen deprivation for advanced prostate cancer: interim analysis of prognostic markers affecting initial response to androgen deprivation. J Urol. 2008;180(3):915–9; discussion 9–20. https://doi.org/10.1016/j.juro.2008.05.009.
Aggarwal RR, Feng FY, Small EJ. Emerging categories of disease in advanced prostate cancer and their therapeutic implications. Oncology (Williston Park). 2017;31(6):467–74.
Ritch CR, Cookson MS. Advances in the management of castration resistant prostate cancer. BMJ. 2016;355:i4405. https://doi.org/10.1136/bmj.i4405.
Shoag J, Barbieri CE. Clinical variability and molecular heterogeneity in prostate cancer. Asian J Androl. 2016;18(4):543–8. https://doi.org/10.4103/1008-682X.178852.
Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101(12):878–87. https://doi.org/10.1093/jnci/djp122.
D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74.
Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153(3):666–77. https://doi.org/10.1016/j.cell.2013.03.021.
Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44(6):685–9. https://doi.org/10.1038/ng.2279.
Taylor BS, Schultz N, Hieronymus H, Gopalan A, **ao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. https://doi.org/10.1016/j.ccr.2010.05.026.
Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448(7153):595–9. https://doi.org/10.1038/nature06024.
Hieronymus H, Schultz N, Gopalan A, Carver BS, Chang MT, **ao Y, et al. Copy number alteration burden predicts prostate cancer relapse. Proc Natl Acad Sci U S A. 2014;111(30):11139–44. https://doi.org/10.1073/pnas.1411446111.
Lalonde E, Ishkanian AS, Sykes J, Fraser M, Ross-Adams H, Erho N, et al. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: a retrospective cohort study. Lancet Oncol. 2014;15(13):1521–32. https://doi.org/10.1016/S1470-2045(14)71021-6.
•• Cancer Genome Atlas Research N. The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011–25. https://doi.org/10.1016/j.cell.2015.10.025. This study provided clinical molecular analysis of early onset prostate cancers and reported the molecular alterations that add heterogeneity observed in patients with primary prostate cancer, which regulates the pathogenesis of this disease.
Borno ST, Fischer A, Kerick M, Falth M, Laible M, Brase JC, et al. Genome-wide DNA methylation events in TMPRSS2-ERG fusion-negative prostate cancers implicate an EZH2-dependent mechanism with miR-26a hypermethylation. Cancer Discov. 2012;2(11):1024–35. https://doi.org/10.1158/2159-8290.CD-12-0041.
Kim JH, Dhanasekaran SM, Prensner JR, Cao X, Robinson D, Kalyana-Sundaram S, et al. Deep sequencing reveals distinct patterns of DNA methylation in prostate cancer. Genome Res. 2011;21(7):1028–41. https://doi.org/10.1101/gr.119347.110.
Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, Rubin MA, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56(2):275–86. https://doi.org/10.1016/j.eururo.2009.04.036.
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–8. https://doi.org/10.1126/science.1117679.
Gasi Tandefelt D, Boormans J, Hermans K, Trapman J. ETS fusion genes in prostate cancer. Endocr Relat Cancer. 2014;21(3):R143–52. https://doi.org/10.1530/ERC-13-0390.
Hermans KG, van der Korput HA, van Marion R, van de Wijngaart DJ, Ziel-van d. Made a, Dits NF et al. truncated ETV1, fused to novel tissue-specific genes, and full-length ETV1 in prostate cancer. Cancer Res. 2008;68(18):7541–9. https://doi.org/10.1158/0008-5472.CAN-07-5930.
Joshua AM, Evans A, Van der Kwast T, Zielenska M, Meeker AK, Chinnaiyan A, et al. Prostatic preneoplasia and beyond. Biochim Biophys Acta. 2008;1785(2):156–81. https://doi.org/10.1016/j.bbcan.2007.12.001.
Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8(7):497–511. https://doi.org/10.1038/nrc2402.
Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29(27):3659–68. https://doi.org/10.1200/JCO.2011.35.1916.
Helgeson BE, Tomlins SA, Shah N, Laxman B, Cao Q, Prensner JR, et al. Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res. 2008;68(1):73–80. https://doi.org/10.1158/0008-5472.CAN-07-5352.
Hermans KG, Bressers AA, van der Korput HA, Dits NF, Jenster G, Trapman J. Two unique novel prostate-specific and androgen-regulated fusion partners of ETV4 in prostate cancer. Cancer Res. 2008;68(9):3094–8. https://doi.org/10.1158/0008-5472.CAN-08-0198.
Lapointe J, Kim YH, Miller MA, Li C, Kaygusuz G, van de Rijn M, et al. A variant TMPRSS2 isoform and ERG fusion product in prostate cancer with implications for molecular diagnosis. Mod Pathol. 2007;20(4):467–73. https://doi.org/10.1038/modpathol.3800759.
Petrovics G, Liu A, Shaheduzzaman S, Furusato B, Sun C, Chen Y, et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene. 2005;24(23):3847–52. https://doi.org/10.1038/sj.onc.1208518.
Wang J, Cai Y, Ren C, Ittmann M. Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res. 2006;66(17):8347–51. https://doi.org/10.1158/0008-5472.CAN-06-1966.
Rajput AB, Miller MA, De Luca A, Boyd N, Leung S, Hurtado-Coll A, et al. Frequency of the TMPRSS2:ERG gene fusion is increased in moderate to poorly differentiated prostate cancers. J Clin Pathol. 2007;60(11):1238–43. https://doi.org/10.1136/jcp.2006.043810.
Nam RK, Sugar L, Yang W, Srivastava S, Klotz LH, Yang LY, et al. Expression of the TMPRSS2:ERG fusion gene predicts cancer recurrence after surgery for localised prostate cancer. Br J Cancer. 2007;97(12):1690–5. https://doi.org/10.1038/sj.bjc.6604054.
Gopalan A, Leversha MA, Satagopan JM, Zhou Q, Al-Ahmadie HA, Fine SW, et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69(4):1400–6. https://doi.org/10.1158/0008-5472.CAN-08-2467.
Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, Stack EC, et al. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomark Prev. 2012;21(9):1497–509. https://doi.org/10.1158/1055-9965.EPI-12-0042.
Shaikhibrahim Z, Wernert N. ETS transcription factors and prostate cancer: the role of the family prototype ETS-1 (review). Int J Oncol. 2012;40(6):1748–54. https://doi.org/10.3892/ijo.2012.1380.
Vitari AC, Leong KG, Newton K, Yee C, O'Rourke K, Liu J, et al. COP1 is a tumour suppressor that causes degradation of ETS transcription factors. Nature. 2011;474(7351):403–6. https://doi.org/10.1038/nature10005.
Tomlins SA, Mehra R, Rhodes DR, Smith LR, Roulston D, Helgeson BE, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66(7):3396–400. https://doi.org/10.1158/0008-5472.CAN-06-0168.
Carver BS, Tran J, Chen Z, Carracedo-Perez A, Alimonti A, Nardella C, et al. ETS rearrangements and prostate cancer initiation. Nature. 2009;457(7231):E1; discussion E2–3–E1; discussion E3. https://doi.org/10.1038/nature07738.
Park K, Tomlins SA, Mudaliar KM, Chiu YL, Esgueva R, Mehra R, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12(7):590–8.
van Leenders GJ, Boormans JL, Vissers CJ, Hoogland AM, Bressers AA, Furusato B, et al. Antibody EPR3864 is specific for ERG genomic fusions in prostate cancer: implications for pathological practice. Mod Pathol. 2011;24(8):1128–38. https://doi.org/10.1038/modpathol.2011.65.
Brase JC, Johannes M, Mannsperger H, Falth M, Metzger J, Kacprzyk LA, et al. TMPRSS2-ERG -specific transcriptional modulation is associated with prostate cancer biomarkers and TGF-beta signaling. BMC Cancer. 2011;11:507. https://doi.org/10.1186/1471-2407-11-507.
Kron KJ, Murison A, Zhou S, Huang V, Yamaguchi TN, Shiah YJ, et al. TMPRSS2-ERG fusion co-opts master transcription factors and activates NOTCH signaling in primary prostate cancer. Nat Genet. 2017;49(9):1336–45. https://doi.org/10.1038/ng.3930.
Baena E, Shao Z, Linn DE, Glass K, Hamblen MJ, Fujiwara Y, et al. ETV1 directs androgen metabolism and confers aggressive prostate cancer in targeted mice and patients. Genes Dev. 2013;27(6):683–98. https://doi.org/10.1101/gad.211011.112.
Aytes A, Mitrofanova A, Kinkade CW, Lefebvre C, Lei M, Phelan V, et al. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proc Natl Acad Sci U S A. 2013;110(37):E3506–15. https://doi.org/10.1073/pnas.1303558110.
• Fisher KW, Montironi R, Lopez Beltran A, Moch H, Wang L, Scarpelli M, et al. Molecular foundations for personalized therapy in prostate cancer. Curr Drug Targets. 2015;16(2):103–14. The study emphasized on the potential clinical utility of genomic profiles as predictive markers for the targeted therapies.
Blattner M, Lee DJ, O'Reilly C, Park K, MacDonald TY, Khani F, et al. SPOP mutations in prostate cancer across demographically diverse patient cohorts. Neoplasia. 2014;16(1):14–20.
Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, et al. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc Natl Acad Sci U S A. 2013;110(17):6997–7002. https://doi.org/10.1073/pnas.1304502110.
Khani F, Mosquera JM, Park K, Blattner M, O'Reilly C, MacDonald TY, et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin Cancer Res. 2014;20(18):4925–34. https://doi.org/10.1158/1078-0432.CCR-13-2265.
Garcia-Flores M, Casanova-Salas I, Rubio-Briones J, Calatrava A, Dominguez-Escrig J, Rubio L, et al. Clinico-pathological significance of the molecular alterations of the SPOP gene in prostate cancer. Eur J Cancer. 2014;50(17):2994–3002. https://doi.org/10.1016/j.ejca.2014.08.009.
An J, Wang C, Deng Y, Yu L, Huang H. Destruction of full-length androgen receptor by wild-type SPOP, but not prostate-cancer-associated mutants. Cell Rep. 2014;6(4):657–69. https://doi.org/10.1016/j.celrep.2014.01.013.
Geng C, Rajapakshe K, Shah SS, Shou J, Eedunuri VK, Foley C, et al. Androgen receptor is the key transcriptional mediator of the tumor suppressor SPOP in prostate cancer. Cancer Res. 2014;74(19):5631–43. https://doi.org/10.1158/0008-5472.CAN-14-0476.
Blattner M, Liu D, Robinson BD, Huang D, Poliakov A, Gao D, et al. SPOP mutation drives prostate tumorigenesis in vivo through coordinate regulation of PI3K/mTOR and AR signaling. Cancer Cell. 2017;31(3):436–51. https://doi.org/10.1016/j.ccell.2017.02.004.
Dai X, Gan W, Li X, Wang S, Zhang W, Huang L, et al. Prostate cancer-associated SPOP mutations confer resistance to BET inhibitors through stabilization of BRD4. Nat Med. 2017;23(9):1063–71. https://doi.org/10.1038/nm.4378.
Zhang P, Wang D, Zhao Y, Ren S, Gao K, Ye Z, et al. Intrinsic BET inhibitor resistance in SPOP-mutated prostate cancer is mediated by BET protein stabilization and AKT-mTORC1 activation. Nat Med. 2017;23(9):1055–62. https://doi.org/10.1038/nm.4379.
Janouskova H, El Tekle G, Bellini E, Udeshi ND, Rinaldi A, Ulbricht A, et al. Opposing effects of cancer-type-specific SPOP mutants on BET protein degradation and sensitivity to BET inhibitors. Nat Med. 2017;23(9):1046–54. https://doi.org/10.1038/nm.4372.
Boysen G, Barbieri CE, Prandi D, Blattner M, Chae SS, Dahija A, et al. SPOP mutation leads to genomic instability in prostate cancer. elife. 2015;4 https://doi.org/10.7554/eLife.09207.
Shenoy TR, Boysen G, Wang MY, Xu QZ, Guo W, Koh FM, et al. CHD1 loss sensitizes prostate cancer to DNA damaging therapy by promoting error-prone double-strand break repair. Ann Oncol. 2017;28(7):1495–507. https://doi.org/10.1093/annonc/mdx165.
Gao N, Zhang J, Rao MA, Case TC, Mirosevich J, Wang Y, et al. The role of hepatocyte nuclear factor-3 alpha (Forkhead box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;17(8):1484–507. https://doi.org/10.1210/me.2003-0020.
Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–43. https://doi.org/10.1038/nature11125.
** HJ, Zhao JC, Ogden I, Bergan RC, Yu J. Androgen receptor-independent function of FoxA1 in prostate cancer metastasis. Cancer Res. 2013;73(12):3725–36. https://doi.org/10.1158/0008-5472.CAN-12-3468.
Prensner JR, Chinnaiyan AM. Metabolism unhinged: IDH mutations in cancer. Nat Med. 2011;17(3):291–3. https://doi.org/10.1038/nm0311-291.
Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–67. https://doi.org/10.1016/j.ccr.2010.11.015.
Kang MR, Kim MS, Oh JE, Kim YR, Song SY, Seo SI, et al. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer. 2009;125(2):353–5. https://doi.org/10.1002/ijc.24379.
Okoye-Okafor UC, Bartholdy B, Cartier J, Gao EN, Pietrak B, Rendina AR, et al. New IDH1 mutant inhibitors for treatment of acute myeloid leukemia. Nat Chem Biol. 2015;11(11):878–86. https://doi.org/10.1038/nchembio.1930.
Yoshimoto M, Cunha IW, Coudry RA, Fonseca FP, Torres CH, Soares FA, et al. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer. 2007;97(5):678–85. https://doi.org/10.1038/sj.bjc.6603924.
Carver BS, Tran J, Gopalan A, Chen Z, Shaikh S, Carracedo A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41(5):619–24. https://doi.org/10.1038/ng.370.
Krohn A, Diedler T, Burkhardt L, Mayer PS, De Silva C, Meyer-Kornblum M, et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am J Pathol. 2012;181(2):401–12. https://doi.org/10.1016/j.ajpath.2012.04.026.
Yoshimoto M, Ding K, Sweet JM, Ludkovski O, Trottier G, Song KS, et al. PTEN losses exhibit heterogeneity in multifocal prostatic adenocarcinoma and are associated with higher Gleason grade. Mod Pathol. 2013;26(3):435–47. https://doi.org/10.1038/modpathol.2012.162.
Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, et al. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96(5):2110–5.
Liu W, Lindberg J, Sui G, Luo J, Egevad L, Li T, et al. Identification of novel CHD1-associated collaborative alterations of genomic structure and functional assessment of CHD1 in prostate cancer. Oncogene. 2012;31(35):3939–48. https://doi.org/10.1038/onc.2011.554.
Huang S, Gulzar ZG, Salari K, Lapointe J, Brooks JD, Pollack JR. Recurrent deletion of CHD1 in prostate cancer with relevance to cell invasiveness. Oncogene. 2012;31(37):4164–70. https://doi.org/10.1038/onc.2011.590.
Brooks JD, Wei W, Hawley S, Auman H, Newcomb L, Boyer H, et al. Evaluation of ERG and SPINK1 by Immunohistochemical staining and Clinicopathological outcomes in a multi-institutional radical prostatectomy cohort of 1067 patients. PLoS One. 2015;10(7):e0132343. https://doi.org/10.1371/journal.pone.0132343.
Tomlins SA, Rhodes DR, Yu J, Varambally S, Mehra R, Perner S, et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell. 2008;13(6):519–28. https://doi.org/10.1016/j.ccr.2008.04.016.
Stenman UH. Tumor-associated trypsin inhibitor. Clin Chem. 2002;48(8):1206–9.
Wang C, Wang L, Su B, Lu N, Song J, Yang X, et al. Serine protease inhibitor Kazal type 1 promotes epithelial-mesenchymal transition through EGFR signaling pathway in prostate cancer. Prostate. 2014;74(7):689–701. https://doi.org/10.1002/pros.22787.
Saad F, Hotte SJ. Guidelines for the management of castrate-resistant prostate cancer. Can Urol Assoc. 2010;4(6):380–4.
•• Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, et al. Prospective genomic profiling of prostate Cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;2017:1–16. https://doi.org/10.1200/PO.17.00029. The study provided clinical genome sequencing from patients at different stages of the disease, and defined how increased burden of genetic alteration dictate prognosis.
Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate Cancer. Eur Urol. 2016;69(6):992–5. https://doi.org/10.1016/j.eururo.2015.11.022.
Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate Cancer. N Engl J Med. 2015;373(18):1697–708. https://doi.org/10.1056/NEJMoa1506859.
Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–9. https://doi.org/10.1038/nm972.
Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. elife. 2013;2:e00499. https://doi.org/10.7554/eLife.00499.
Duff J, McEwan IJ. Mutation of histidine 874 in the androgen receptor ligand-binding domain leads to promiscuous ligand activation and altered p160 coactivator interactions. Mol Endocrinol. 2005;19(12):2943–54. https://doi.org/10.1210/me.2005-0231.
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–38. https://doi.org/10.1056/NEJMoa1315815.
Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–77. https://doi.org/10.1158/0008-5472.CAN-08-0594.
Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54(20):5474–8.
Nelson EC, Evans CP, Mack PC, Devere-White RW, Lara PN Jr. Inhibition of Akt pathways in the treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2007;10(4):331–9. https://doi.org/10.1038/sj.pcan.4500974.
Parimi V, Goyal R, Poropatich K, Yang XJ. Neuroendocrine differentiation of prostate cancer: a review. Am J Clin Exp Urol. 2014;2(4):273–85.
Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38(6):756–67. https://doi.org/10.1097/PAS.0000000000000208.
Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1(6):487–95. https://doi.org/10.1158/2159-8290.CD-11-0130.
Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34(7):646–53.
Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64(24):9209–16. https://doi.org/10.1158/0008-5472.CAN-04-2442.
Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, et al. Androgen receptor pathway-independent prostate Cancer is sustained through FGF signaling. Cancer Cell. 2017;32(4):474–89 e6. https://doi.org/10.1016/j.ccell.2017.09.003.
Acknowledgements
We are grateful to the individuals with prostate cancer and their families for their contributions. This work was supported by: US NCI (K08CA187417-01, C.E.B., P50CA211024-01, C.E.B., and R01CA215040-01, C.E.B.), Urology Care Foundation Rising Star in Urology Research Award (C.E.B.), and Damon Runyon Cancer Research Foundation MetLife Foundation Family Clinical Investigator Award (C.E.B.), and the Prostate Cancer Foundation.
Author information
Authors and Affiliations
Contributions
KA and CEB reviewed the relevant literature and wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Kaveri Arora and Christopher E. Barbieri declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Arora, K., Barbieri, C.E. Molecular Subtypes of Prostate Cancer. Curr Oncol Rep 20, 58 (2018). https://doi.org/10.1007/s11912-018-0707-9
Published:
DOI: https://doi.org/10.1007/s11912-018-0707-9