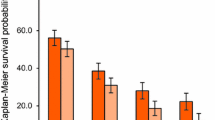
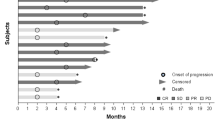
Abstract
Aflibercept (known as ziv-aflibercept in the USA and sold under the trade name Zaltrap®) is a human recombinant fusion protein with antiangiogenic effects that functions as a decoy receptor to bind vascular endothelial growth factors A and B and placental growth factor. Its unique mechanism of action with respect to other agents targeting angiogenesis led investigators to speculate that it may be more ubiquitously efficacious in tumors highly dependent on pathologic angiogenesis for their growth. Despite encouraging preclinical studies in various tumor types, aflibercept has not been proven efficacious in most later-phase clinical studies. In fact, its only currently held US Food and Drug Administration indication is in metastatic colorectal cancer in combination with 5-fluorouracil, leucovorin, and irinotecan for those patients previously treated with an oxaliplatin-containing chemotherapy regimen. Given aflibercept’s toxicity profile and cost, further investigation is needed to better understand its mechanism of action and to discover predictive biomarkers for optimization of its appropriate use in treatment of cancer patients.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307.
Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–93.
Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–91.
Holash J et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–8.
Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12(6):933–41.
Lohela M et al. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21(2):154–65.
Fischer C et al. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8(12):942–56.
Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82(3):673–700.
Park JE et al. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269(41):25646–54.
Holash J et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–8.
Willett CG et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27(18):3020–6.
Rini BI et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26(22):3743–8.
Baffert F et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290(2):H547–59.
Fukasawa M, Korc M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res. 2004;10(10):3327–32.
Verheul HM et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res. 2007;13(14):4201–8.
Le XF et al. Specific blockade of VEGF and HER2 pathways results in greater growth inhibition of breast cancer xenografts that overexpress HER2. Cell Cycle. 2008;7(23):3747–58.
Hu L et al. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res. 2005;11(19 Pt 1):6966–71.
Cheron M VP, L.P., Demers B, Leopold D, Bissery MC. Synergistic activity of aflibercept (VEGF Trap) in combination with 5-fluorouracil and irinotecan in preclinical tumor models: abstract A13. In: Proceedings from the AACR-NCI-EORTC: molecular targets and cancer therapeutics, 2007
Tew WP et al. Phase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumors. Clin Cancer Res. 2010;16(1):358–66.
Lockhart AC et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28(2):207–14. This phaseI trial of intravenously administered aflibercept established its safety and toxicity profile, as well as its recommended phase II dose.
Khayat D et al. Intravenous aflibercept administered in combination with irinotecan, 5-fluorouracil and leucovorin in patients with advanced solid tumours: results from the expansion cohort of a phase I study. Eur J Cancer. 2013;49(4):790–7.
Isambert N et al. Phase I dose-escalation study of intravenous aflibercept in combination with docetaxel in patients with advanced solid tumors. Clin Cancer Res. 2012;18(6):1743–50.
Diaz-Padilla I et al. A phase I dose-escalation study of aflibercept administered in combination with pemetrexed and cisplatin in patients with advanced solid tumours. Br J Cancer. 2012;107(4):604–11.
Van Cutsem E et al. Phase I dose-escalation study of intravenous aflibercept administered in combination with irinotecan, 5-fluorouracil and leucovorin in patients with advanced solid tumours. Eur J Cancer. 2013;49(1):17–24.
Gaya A, Tse V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat Rev. 2012;38(5):484–93. This excellent review comprehensively discusses the preclinical and clinical development of aflibercept.
Tang PA et al. Phase II clinical and pharmacokinetic study of aflibercept in patients with previously treated metastatic colorectal cancer. Clin Cancer Res. 2012;18(21):6023–31.
Pericay CFG, Saunders M, Thomas A, Roh JK, Lopez R, et al. Phase 2 randomized, noncomparative open-label study of aflibercept and modified FOLFOX6 in the first line treatment of metastatic colorectal cancer (AFFIRM): abstract O = 0024. Ann Oncol. 2012;23 Suppl 4:iv5–18.
Ramlau R et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol. 2012;30(29):3640–7.
Rougier P et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49(12):2633–42.
Tannock IF et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol. 2013;14(8):760–8.
Van Cutsem E et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–506. This pivotal phase III trial demonstrated a survival benefit of aflibercept in combination with FOLFIRI for patients with oxaliplatin-pretreated metastatic CRC and led to its FDA approval for this indication.
Hurwitz H et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89.
Bennouna J et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. This phase III trial demonstrated the survival benefit of continuing bevacizumab therapy in the second-line setting beyond progression with bevacizumab therapy in the first-line setting for metastatic CRC.
Grothey A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12. This phaseIII trial showed a survival benefit of single-agent regorafenib for patients with refractory metastatic CRC and led to its FDA approval for this indication.
Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times. 2012 Oct 15:A25.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Kristen K. Ciombor has served as a consultant for Bayer, has received travel/accommodation expenses reimbursed by Bayer, and has received honoraria from Clinical Advances in Hematology and Oncology.
Jordan Berlin has served on the Scientific Advisory Board of Amgen and on the Advisory Board of Genentech/Roche.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Evolving Therapies
Rights and permissions
About this article
Cite this article
Ciombor, K.K., Berlin, J. Aflibercept—a Decoy VEGF Receptor. Curr Oncol Rep 16, 368 (2014). https://doi.org/10.1007/s11912-013-0368-7
Published:
DOI: https://doi.org/10.1007/s11912-013-0368-7