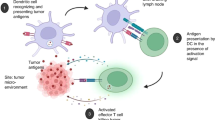
Abstract
Allogeneic hematopoietic stem cell transplant (HSCT) relies primarily upon graft-versus-tumor activity for cancer eradication. Relapse remains the principal cause of treatment failure after HSCT, implying frequent immune escape, which in at least some cases, appears to be mediated by increased expression of inhibitory immune checkpoints. In an attempt to restore anti-tumor immunity, checkpoint blockade therapy (CBT) targeting PD-1 and CLTA-4 has been used in conjunction with both allogeneic and autologous HSCT. Clinical experience in this setting is limited to several small clinical trials and case series, but together they suggest that treatment with CBT can effectively amplify anti-tumor immune responses. However, intrinsic to its mechanism is also the risk that CBT in the HSCT setting may also cause significant immune toxicity. Fatal immune-related adverse events and graft-versus-host disease have been observed, but in most cases, immune side effects appear to be reversible with steroids and CBT discontinuation. As clinical investigation continues, improved understanding of immune checkpoint biology will be critical to optimize safe and efficacious treatment strategies.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
Norde WJ, Maas F, Hobo W, et al. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011;71(15):5111–22.
Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13.
Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England). 2016;387(10031):1909–20.
Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016.
Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15(20):6446–53.
Roemer MGM, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34(23):2690–7.
• Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2014;372(4):311–9. PD-1 blockade in Hodgkin Lymphoma is well-tolerated with a high overall response rate and durable remissions.
Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016.
Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–94.
Lesokhin AM, Ansell SM, Armand P, Scott ECHA. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698–704.
Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015;125(22):3393–400.
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88.
Porrata LF, Litzow MR, Markovic SN. Immune reconstitution after autologous hematopoietic stem cell transplantation. Mayo Clin Proc. 2001;76(4):407–12.
Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199–206.
• Merryman RW, Kim HT, Zinzani PL, et al. Safety and efficacy of allogeneic hematopoetic stem cell transplant (HSCT) after treatment with programmed cell death 1 (PD-1) inhibitors. Blood. 2015;126(23). Allo-HSCT after PD-1 blockade is feasible but may be associated with increased early immune toxicities.
Armand P, Kim HT, Sainvil M-M, et al. The addition of sirolimus to the graft-versus-host disease prophylaxis regimen in reduced intensity allogeneic stem cell transplantation for lymphoma: a multicentre randomized trial. Br J Haematol. 2016;173(1):96–104.
Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127(7):938–47.
Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–71.
Alyea EP, Kim HT, Ho V, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105(4):1810–4.
Antin JH. Graft-versus-leukemia: no longer an epiphenomenon. Blood. 1993;82(8):2273–7.
• Kong Y, Zhang J, Claxton DF, et al. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation. Blood Cancer J. 2015;5:e330. Inhibitory checkpoint receptors are upregulated on T cells in patients who relapse after allo-HSCT and may predict relapse several months before clinically apparent recurrence.
Schnorfeil FM, Lichtenegger FS, Emmerig K, et al. T cells are functionally not impaired in AML: increased PD-1 expression is only seen at time of relapse and correlates with a shift towards the memory T cell compartment. J Hematol Oncol. 2015;8:93.
Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sharpe AH, Vallera DA. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J Immunol. 1999;162(11):6368–77.
Fevery S, Billiau AD, Sprangers B, et al. CTLA-4 blockade in murine bone marrow chimeras induces a host-derived antileukemic effect without graft-versus-host disease. Leukemia. 2007;21(7):1451–9.
Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113(7):1581–8.
•• Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375(2):143–53. Blockade of CTLA-4 with ipilimumab resulted in durable responses in patients with various hematologic malignancies who had relapsed after allogeneic HSCT. It is the largest prospective trial to date showing that immune checkpoint blockade is a viable strategy for relapse after allo-HSCT.
Schade H, Sen S, Neff CP, et al. Programmed death 1 expression on CD4(+) T cells predicts mortality after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2016.
• Krönig H, Kremmler L, Haller B, et al. Interferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatment. Eur J Haematol. 2014;92(3):195–203. Alterations in expression of PD-1 and its ligands appears to be an important mechanism of tumor immune escape.
• Michonneau D, Sagoo P, Breart B, et al. The PD-1 axis enforces an anatomical segregation of CTL activity that creates tumor niches after allogeneic hematopoietic stem cell transplantation. Immunity. 2016;44(1):143–54. In a mouse model, differential upregulation of PD-1 ligand resulted in a compartmentalized GVT response and the emergence of niches for tumor immune escape. PD-1 blockade restored the efficacy of GVT across these anatomic niches.
Koestner W, Hapke M, Herbst J, et al. PD-L1 blockade effectively restores strong graft-versus-leukemia effects without graft-versus-host disease after delayed adoptive transfer of T-cell receptor gene-engineered allogeneic CD8+ T cells. Blood. 2011;117(3):1030–41.
Blazar BR, Carreno BM, Panoskaltsis-Mortari A, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171(3):1272–7.
Fujiwara H, Maeda Y, Kobayashi K, et al. Programmed death-1 pathway in host tissues ameliorates Th17/Th1-mediated experimental chronic graft-versus-host disease. J Immunol. 2014;193(5):2565–73.
Amarnath S, Mangus CW, Wang JCM, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3(111):111–20.
Asano T, Kishi Y, Meguri Y, et al. PD-1 signaling has a critical role in maintaining regulatory T cell homeostasis; implication for Treg depletion therapy by PD-1 blockade. Blood. 2015;126(23):848.
Khan AR, Hams E, Floudas A, et al. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun. 2015;6:5997.
Saha A, Aoyama K, Taylor PA, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122(17):3062–73.
Saha A, O’Connor RS, Thangavelu G, et al. Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality. J Clin Invest. 2016;126(7):2642–60.
Villasboas JC, Ansell SM, Witzig TE. Targeting the PD-1 pathway in patients with relapsed classic Hodgkin lymphoma following allogeneic stem cell transplant is safe and effective. Oncotarget. 2016;7(11):13260–4.
Kwong Y-L. Safety of pembrolizumab after allogeneic haematopoietic stem cell transplantation. Ann Hematol. 2016;95(7):1191–2.
Chan TSY, Khong P-L, Kwong Y-L. Pembrolizumab for relapsed anaplastic large cell lymphoma after allogeneic haematopoietic stem cell transplantation: efficacy and safety. Ann Hematol. 2016.
Singh AK, Porrata LF, Aljitawi O, et al. Fatal GvHD induced by PD-1 inhibitor pembrolizumab in a patient with Hodgkin’s lymphoma. Bone Marrow Transplant. 2016;51(9):1268–70.
Herbaux C, Gauthier J, Brice P, et al. Nivolumab is effective and reasonably safe in relapsed or refractory Hodgkin’s lymphoma after allogeneic hematopoietic cell transplantation: a study from the Lysa and SFGM-TC. Blood. 2015;126(23):3979.
Yared JA, Hardy N, Singh Z, et al. Major clinical response to nivolumab in relapsed/refractory Hodgkin lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(6):850–2.
Angenendt L, Schliemann C, Lutz M, et al. Nivolumab in a patient with refractory Hodgkin’s lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(3):443–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Reid Merryman declares no potential conflicts of interest.
Philippe Armand is a consultant for BMS, Merck, and Infinity and reports research funding from BMS, Merck, Pfizer, Affimed, and Roche.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Stem Cell Transplantation
Rights and permissions
About this article
Cite this article
Merryman, R.W., Armand, P. Immune Checkpoint Blockade and Hematopoietic Stem Cell Transplant. Curr Hematol Malig Rep 12, 44–50 (2017). https://doi.org/10.1007/s11899-017-0362-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-017-0362-5