Abstract
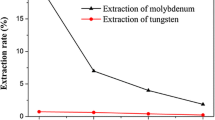
A solvent extraction process for separation of tungsten from phosphorus-containing molybdate solution was developed using acidified primary amine N1923 as extractant. Most tungsten combines with phosphorus to form phosphorus-containing polymeric ions over a proper pH range, while the dominant form of molybdenum is still monomeric ions (MoO42−). The former can be preferentially extracted by acidified N1923. The initial pH, extractant concentration, 2-octanol concentration, and temperature were optimized. By employing an initial pH of 7.5, 15% by volume N1923, 5% by volume 2-octanol, T = 20°C, and O:A ratio of 1:1, 97.0% tungsten was extracted in a single stage, while limiting molybdenum coextraction to 12.5%. The concentration of WO3 in the raffinate was reduced to 0.28 g/L after two-stage continuous countercurrent extraction.






Similar content being viewed by others
References
C.K. Gupta, Extractive Metallurgy of Molybdenum (Florida: CRC Press, 1992), pp. 143–146.
A.G. Kholmogorov and O.N. Kononova, Hydrometallurgy 76, 37 (2005).
Y. Zhao and J. Chen, Hydrometallurgy 42, 325 (1996).
R.R. Srivastava, N.K. Mittal, B. Padh, and B.R. Reddy, Hydrometallurgy 127–128, 77 (2012).
Y. Song, L. He, X. Chen, and Z. Zhao, Trans. Nonferrous Met. Soc. China 27, 2492 (2017).
X. Chen, X. Liu, J. Liu, and Z. Zhao, Int. J. Refract. Met. Hard Mater. 61, 259 (2016).
Y. Song, X. Chen, Z. Zhao, J. Zhang, and L. He, Metall. Mater. Trans. B 47, 675 (2016).
C. Cao, Z. Zhao, and X. Chen, Hydrometallurgy 110, 115 (2011).
G. Huo, C. Peng, Q. Song, and X. Lu, Hydrometallurgy 147–148, 217 (2014).
X. Lu, G. Huo, and C. Liao, Trans. Nonferrous Met. Soc. China 24, 3008 (2014).
Z. Zhao, J. Zhang, X. Chen, X. Liu, J. Li, and W. Zhang, Hydrometallurgy 140, 120 (2013).
P. Ning, H. Cao, and Y. Zhang, Sep. Purif. Technol. 70, 27 (2009).
Z. Li, G. Zhang, W. Guan, L. Zeng, L. **ao, Q. Li, Z. Cao, and X. Lu, Hydrometallurgy 175, 203 (2018).
Y. Sasaki, M. Hennichs, P.M. Jørgensen, S. Refn, V.K. Andersen, and A. Jart, Acta Chem. Scand. 15, 175 (1961).
J. Li and Z. Zhao, Hydrometallurgy 163, 55 (2016).
B.J. Smith, V.A. Patrick, B.J. Smith, and V.A. Patrick, Aust. J. Chem. 57, 261 (2004).
L. Pettersson, I. Andersson, and L.O. Oehman, Inorg. Chem. 25, 4726 (1986).
Acknowledgements
The authors express their sincere thanks to the Natural Science Foundation of China (NSFC, No. 51334008) and Postdoctoral Science Foundation of Central South University, China for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, H., Li, Y., Cao, G. et al. Separation of Tungsten from Phosphorus-Containing Molybdate Solution Using Solvent Extraction with Primary Amine N1923. JOM 70, 2864–2868 (2018). https://doi.org/10.1007/s11837-018-3167-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-3167-7