Abstract
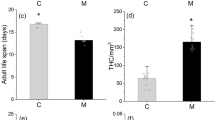
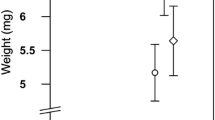
In this study we tested the effects of rapid induced resistance of the silver birch, Betula pendula, on the performance and immune defense of the gypsy moth, Lymantria dispar. We also measured the effects of defoliation on the concentrations of plant secondary metabolites, particularly on phenolics and terpenoids. It was found that severe natural defoliation (by moth larvae) of silver birch led to an increase in lipophilic flavonoids on the leaf surface. The concentration of some simple phenolics and monoterpenes (linalool and geraniol) also increased, while that of several glycosides of quercetin decreased. The female pupal weights and survival rates of moths decreased, and larval development time increased, when the insects fed on defoliated trees. However, the feeding of caterpillars with the leaves of defoliated trees led to an increase in lysozyme-like activity in their hemolymph, with an increase in their ability to encapsulate potential parasites. Our data show that the silver birch deploys a rapid chemical defense against gypsy moth larvae. We suggest that lipophilic flavonoids are important compounds in the direct silver birch defense against L. dispar caterpillars. The increased strength of immune defense of insects exposed to trees that had deployed a rapid induced resistance may be an adaptation of the herbivores to resist the rising density of parasites when host population density is high.



Similar content being viewed by others
References
Barbehenn RV, Jaros A, Lee G, Mozola C, Weir Q, Salminen J-P (2009) Hydrolyzable tannins as “quantitative defenses”: limited impact against Lymantria dispar caterpillars on hybrid poplar. J Insect Physiol 55:297–304
Berenbaum MR (1995) Turnabout is fair play: secondary roles for primary compounds. J Chem Ecol 21:925–940
Bradford JM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Doane CC, McManus ML (1981) The gypsy moth: research toward integrated pest management. Department of Agriculture, Forest Service, Science and Education Agency. Animal and Plant Health Inspection Service, Washington, USA
Doskotch RW, Cheng H-Y, Odell TM, Girard L (1980) Nerolidol: an antifeeding sesquiterpene alcohol for gypsy moth larvae from Melaleuca leucadendron. J Chem Ecol 6:845–851
Dubovskiy IM, Krukova NA, Glupov VV (2008) Phagocytic activity and encapsulation rate of Galleria mellonella larvae hemocytes during bacterial infection by Bacillus thuringiensis. J Invert Pathol 98:360–362
Haukioja E (1991) Induction of defenses in trees. Ann Rev Entomol 36:25–42
Haukioja E (2005) Plant defenses and population fluctuations of forest defoliators: mechanism-based scenarios. Ann Zool Fenn 42:313–325
Kaitaniemi P, Ruohomäki K, Ossipov V, Haukioja E, Pihlaja K (1998) Delayed induced changes in the biochemical composition of host plant leaves during an insect outbreak. Oecologia 116:182–190
Kapari L, Haukioja E, Rantala MJ, Ruuhola T (2006) Defoliating insect immune defense interacts with induced plant defense during a population outbreak. Ecology 87:291–296
Keane S, Ryan MF (1999) Purification, characterization, and inhibition by monoterpenes of acetylcholinesterase from the wax moth, Galleria mellonella (L.). Insect Biochem Mol Biol 29:1097–1104
Keinänen M, Julkunen-Tiitto R, Mutikainen P, Walls M, Ovaska J, Vapaavuori E (1999) Trade-offs in phenolic metabolism of silver birch: Effects of fertilization, defoliation, and genotype. Ecology 80:1970–1986
Keinänen M, Julkunen-Tiitto R (1996) Effect of sample preparation method on birch (Betula pendula Roth) leaf phenolics. J Agric Food Chem 44:2724–2727
Keinänen M, Julkunen-Tiitto R (1998) High-performance liquid chromatographic determination of flavonoids in Betula pendula and Betula pubescens leaves. J Chromatogr A 793:370–377
Lahtinen M, Salminen J-P, Kapari L, Lempa K, Ossipov V, Sinkkonen J, Valkama E, Haukioja E, Pihlaja K (2004) Defensive effect of surface flavonoid aglycones of Betula pubescens leaves against first-instar Epirrita autumnata larvae. J Chem Ecol 30:2257–2268
Lahtinen M, Lempa K, Salminen J-P, Pihlaja K (2006) HPLC analysis of leaf surface flavonoids for the preliminary classification of birch species Phytochem Anal 17:197–203
Larsson S (2002) Resistance in trees to insects—an overview of mechanisms and interactions. In: Wagner MR et al (eds) Mechanisms and deployment of resistance in trees to insects. Kluwer Academic Publishers, Netherlands, pp 1–29
Lazarević J, Perić-Mataruga V, Stojković B, Tucić N (2002) Adaptation of the gypsy moth to an unsuitable host plant. Entomol Exp App 102:75–86
Lee KP, Cory JS, Wilson K, Raubenheimerand D, Simpson SJ (2006) Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc R Soc London Ser B 273:823–829
Lempa K, Agrawal AA, Salminen J-P, Turunen T, Ossipov V, Ossipova S, Haukioja E, Pihlaja K (2004) Rapid herbivore-induced changes in mountain birch phenolics and nutritive compounds and their effects on performance of the major defoliator, Epirrita autumnata. J Chem Ecol 30:303–321
Likens ST, Nickerson GB (1964) Detection of certain hop oil constituents in brewing products. Proc Am Brewing Chem 5:5–13
Martemyanov VV, Bakhvalov SA (2007) Interrelationships of plant–insect–parasite systems and their influence on the development and population dynamics of forest defoliators. Eurasian Entomol J 6:205–221
Martemyanov VV, Bakhvalov SA, Dubovskiy IM, Glupov VV, Salakhutdinov NF, Tolstikov GA (2006) Effect of tannic acid on the development and resistance of the gypsy moth Lymantria dispar L. to viral infection. Dokl Biochem Biophys 409:219–222
Martemyanov VV, Dubovskiy IM, Rantala, MJ, Salminen JP, Belousova IA, Pavlushin SV, Bakhvalov SA, Glupov VV (2012) The effects of defoliation-induced delayed changes in silver birch foliar chemistry on gypsy moth fitness, immune response, and resistance to baculovirus infection. J Chem Ecol 38:295–305
Mutikainen P, Walls M, Ovaska J, Keinanen M, Julkunen-Tiitto R, Vapaavuori E (2000) Herbivore resistance in Betula pendula: effect of fertilization, defoliation, and plant genotype. Ecology 81:49–65
Neuvonen S, Haukioja E (1991) The effects of inducible resistance in host foliage on birch-feeding herbivores. In: Tallamy DW, Raupp MJ (eds) Phytochemical induction by herbivores. Wiley, New York, pp 277–291
Ojala K, Julkunen-Tiitto R, Lindström L, Mappes J (2005) Diet affects the immune defence and life-history traits of an Arctiid moth Parasemia plantaginis. Evol Ecol Res 7:1153–1170
Osier TL, Lindroth RL (2001) Effects of genotype, nutrient availability, and defoliation on aspen phytochemistry and insect performance. J Chem Ecol 27:1289–1313
Ossipov V, Nurmi K, Loponen J, Haukioja E, Pihlaja K (1996) HPLC separation and identification of phenolic compounds from leaves of Betula pubescens and Betula pendula. J Chromatogr A 721:59–68
Ossipov V, Haukioja E, Ossipova S, Hanhimäki S, Pihlaja K (2001) Phenolic and phenolic-related factors as determinants of suitability of mountain birch leaves to an herbivorous insect. Biochem Syst Ecol 29:223–240
Parry D, Herms DA, Mattson WJ (2003) Responses of an insect folivore and its parasitoids to multiyear experimental defoliation of aspen. Ecology 84:1768–1783
Rantala MJ, Roff DA (2007) Inbreeding and extreme outbreeding causes sex differences in immune defence and life history traits in Epirrita autumnata. Heredity 98:329–336
Roden DB, Mattson WJ (2008) Rapid induced resistance and host species effects on gypsy moth, Lymantria dispar (L.): implications for outbreaks on three tree species in the boreal forest. For Ecol Manag 255:1868–1873
Rosenthal GA, Berenbaum MR (1992) Herbivores, their interactions with secondary plant metabolites. The chemical participants, vol I. Academic Press, New York
Ruuhola T, Yang S, Rantala MJ (2010) Increase in substrate availability down-regulates PO activity in Epirrita autumnata. Chemoecology 20:11–18
Ryan MF, Byrne O (1988) Plant-insect coevolution and inhibition of acetylcholinesterase. J Chem Ecol 14:1965–1975
Salminen J-P, Ossipov V, Loponen J, Haukioja E, Pihlaja K (1999) Characterisation of hydrolysable tannins from leaves of Betula pubescens by high-performance liquid chromatography–mass spectrometry. J Chromatogr A 864:283–291
Siva-Jothy MT, Thompson JW (2002) Short-term nutrient deprivation affects immune function. Physiol Entomol 27:206–212
Smilanich AM, Dyer LE, Gentry GL (2009) The insect immune response and other putative defenses as effective predictors of parasitism. Ecology 90:1434–1440
Stevens MT, Lindroth RL (2005) Induced resistance in the indeterminate growth of aspen (Populus tremuloides). Oecologia 145:298–306
Stockhoff BA (1993) Ontogenetic change in dietary selection for protein and lipid by gypsy moth larvae. J Insect Physiol 39:677–686
Tkachev AV (2008) The investigation of plant volatiles. Izdatelsko-poligraficheskoe predprijatie “Ofset”. Novosibirsk, Russia
Trudeau D, Washburn JO, Volkman LE (2001) Central role of hemocytes in Autographa californica M nucleopolyhedrovirus pathogenesis in Heliothis virescens and Helicoverpa zea. J Virol 75:996–1003
Valkama E, Salminen J-P, Koricheva J, Pihlaja K (2003) Comparative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Ann Bot 91:643–655
Valkama E, Salminen JP, Koricheva J, Pihlaja K (2004) Changes in leaf trichomes and epicuticular flavonoids during leaf development in three birch taxa. Ann Bot 94:233–242
Valkama E, Koricheva J, Salminen J-P, Helander M, Saloniemi I, Saikkonen K, Pihlaja K (2005) Leaf surface traits: overlooked determinants of birch resistance to herbivores and foliar microfungi? Trees 19:191–197
Vuorinen T, Nerg A-M, Syrjälä L, Peltonen P, Holopainen JK (2007) Epirrita autumnata induced VOC emission of silver birch differ from emission induced by leaf fungal pathogen. Arthropod-Plant Interact 1:159–165
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Wilson K, Cotter ShC, Reeson AF, Pell JuK (2001) Melanism and disease resistance in insects. Ecol Lett 4:637–649
Yang S, Ruuhola T, Rantala MJ (2007) Impacts of starvation on immune defense and other life history traits of an outbreaking geometrid, Epirrita autumnata: a possible ultimate trigger of the crash phase of population cycle. Ann Zool Fenn 44:89–96
Acknowledgments
We thank Alexey Tkachev for help in phytochemical assay and for his comments on the earlier version of the manuscript and thank Derek Roff and Will Sillitoe for the language correction. We also thank Ekaterina Chertkova for her assistance in the study of insect immunecometens, and Elena Shatalova, Elena Bojarischeva, and Ekatirina Grizanova for their help in field work. The study was financially supported by a Kone Foundation grant for V. Martemyanov, by a grant from the Russian Foundation for Basic Research (grant Nr 07-04-00870), and by a grant of the President of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martemyanov, V.V., Dubovskiy, I.M., Belousova, I.A. et al. Rapid induced resistance of silver birch affects both innate immunity and performance of gypsy moths: the role of plant chemical defenses. Arthropod-Plant Interactions 6, 507–518 (2012). https://doi.org/10.1007/s11829-012-9202-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-012-9202-7