Abstract
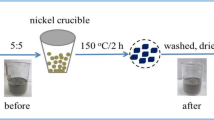
Power and desalination plants are one of the main anthropogenic sources for CO2 generation, which is one of the key elements to cause greenhouse gas effect and thus contribute to the global warming. Fly ash (FA) generated in desalination and power plants was converted into activated carbon (AC) treated with KOH at higher temperature and tested for CO2 capturing efficiency. Morphological characteristics of FA such as BET specific surface area (SSA), pore volume, pore diameter, and pore size distribution (PSD) were performed using N2 adsorption isotherm. CO2 adsorption capacity and adsorption isotherms of CO2 over AC were measured by performing thermogravimetric analysis at different temperatures. BET SSA of 161 m2g−1 and adsorption capacity of 26mg CO2/g AC can be obtained by activation at KOH/FA ratio of 5 at 700 °C and activation time of 2 h. Therefore, great potential exists for producing AC from FA, which will have the positive effect of reducing the landfill problem and global warming.
Similar content being viewed by others
References
M. M. M. Valer, C. Song and Y. Soong, Environmental challenges and greenhouse gas Control for fossil fuel utilization in the 21 st Century, Springer (2003).
C. L. Quere, Earth Syst. Sci. Data, 6, 689 (2013).
M. Daous, The 20 th International conference on solid waste technology and management, Philadelphia, USA (2005).
M.M. Rahman, A.G. Dalvi, K.A. Rabbani, S. Al-Sulami, F. Mandili, H. M. Khaledi and B. Al-Jowdi, Evaluation of fuel chemical additives to reduce corrosion and stack emission in SWCC power plants, 4 th SWCC Acquired Experience Symposium, Jeddah, Saudi Arabia (2005).
S. Wang and H. Wu, J. Hazard. Mater. B, 136, 482 (2006).
D. C. Adriano, A. L. Page, A. A. Elseewi, A. C. Chang and I. Straughan, J. Environ. Qual., 9, 333 (1980).
J. Paya, J. Monzo, M.V. Borrachero, E. Peris and E.G. Lopez, Cem. Concr. Res., 27, 1365 (1997).
K.D. Weerdt, M. B. Haha, G. L. Saout, K.O. Kjellsen, H. Justnes and B. Lothenbach. Cem. Concr. Res., 41, 279 (2011).
R. J. Flatt, N. Roussel and C.R. Cheeseman, J. Eurp. Ceram. Soc., 32, 2787 (2012).
N. Sombatsompop, S. Thongsang, T. Markpin and E. Wimolmala, J. Appl. Polym. Sci., 93, 2119 (2004).
P. Chindaprasirt, P.D. Silva, K. Sagoe-Crentsil and S. Hanjitsuwan, J. Mater. Sci., 47, 4876 (2012).
D.P. Vargas, L. Giraldo and J. C. M. Piraján, Int. J. Mol. Sci., 13, 8388 (2012).
D.M. D. Alessandro, B. Smit and J.R. Long, Angew. Chem. Int. Ed., 49, 6058 (2010).
C. H. Yu, C. H. Huang and C. S. Tan, Aerosol Air Qual. Res., 12, 745 (2012).
R. C. Bansal, J.B. Donnet, F. Stoeckli and M. Deckker, J. Dispersion Sci. Technol., 11, 323 (1990).
M. Valix, W. H. Cheung and G. McKay, Chemosphere, 56, 493 (2004).
T. H. Usmani, M. Tahir, I. Siddiqui and F. A. Parveen, J. Chem. Soc. Pak., 25, 183 (2003).
F. Caturla, M. M. Sabio and F.R. Reinoso, Carbon, 29, 999 (1991).
A. A. Pour and D.D. Do, Carbon, 34, 471 (1996).
K. Somna, C. Jaturapitakkul, P. Kajitvichyanukul and P. Chindaprasirt, Fuel, 90, 2118 (2011).
F. C. Wu, P.H. Wu, R. L. Tseng and R. S. Juang, J. Environ. Manage., 91, 1097 (2010).
R.L. Tseng, S. K. Tseng, F. C. Wu, C. C. Hu and C.C. Wang, J. Chin. Inst. Chem. Eng., 39, 37 (2008).
M. A. L. Rodenas, J. J. Juan, D. C. Amoros and A. L. Solano, Carbon, 42, 1371 (2004).
M. A. L. Rodenas, D. C. Amoros and A. L. Solano, Carbon, 41, 267 (2003).
G. G. Stavropoulos, Fuel Process. Technol., 86, 1165 (2005).
L. Chunlan, X. Shao**, G. Yixiong, L. Shuqin and L. Changhou, Carbon, 43, 2295 (2005).
S. Lowell, J. E. Shields, M. A. Thomas and M. Thommes, Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density, Kluwer Academic Publishers, Netherland (2004).
T. Yang and A. C. Lua, J. Colloid Interface Sci., 267, 408 (2003).
M. J. I. Gómez, A. G. García, C. S. M. de Lecea and A. L. Solano, Energy Fuels, 10, 1108 (1996).
F. Rouquerol, J. Rouquerol and K. Sing, Adsorption by powders and porous solids: Principles methodology and applications, Academic Press, London (1999).
G. Horvath and K. Kawazoe, J. Chem. Eng. Jpn., 16, 470 (1983).
J.A.M. Agullo, B.C. Moore, D.C. Amoros and A.L. Solano, Micropor. Mesopor. Mater., 101, 397 (2007).
Allwar,_A.M. Noor and M.A.M. Nawi, J. Phys. Sci., 19(2), 93 (2008).
G. Aranovich and M. Donohue, J. Colloid Interface Sci., 194, 392 (1997).
S. H. Yoon, S. Lim, Y. Song, Y. Ota, W. Qiao, A. Tanaka and I. Mochida, Carbon, 42, 1723 (2004).
F. Rouquerol, J. Rouquerol and K. Sing, Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, First edition, Academic Press (1998).
R.T. Yang, Gas separation by adsorption process, vol. 1, Imperial College Press, Boston (1997).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Alhamed, Y.A., Rather, S.U., El-Shazly, A.H. et al. Preparation of activated carbon from fly ash and its application for CO2 capture. Korean J. Chem. Eng. 32, 723–730 (2015). https://doi.org/10.1007/s11814-014-0273-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0273-2