Abstract
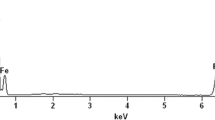
Phosphate is a major pollutant in water, causing serious environmental and health consequences. In present study, the phosphate adsorption on novel magnetite-enriched particles (MEP) was comprehensively investigated. A new method and device were introduced for the separation of MEP from the mill scale at low magnetic intensity. Particles were characterized with different techniques such as XRD, XRF, SEM and EDS. The XRD and XRF analysis of MEP identified the dominant existence of crystalline magnetite. Furthermore, the morphological analysis of MEP confirmed the agglomerate porous morphology of magnetite. Oxygen and iron, the main constituents of magnetite were acknowledged during the elemental analysis using EDS. The phosphate adsorption on MEP is well explained using various isotherm and kinetic models, exhibiting the monolayer adsorption of phosphate on the surface of MEP. The maximum adsorption capacity was determined 6.41 mg/g. Based on particle size (45-75 and 75-150 μm) and empty bed contact time (1 and 2 h), four columns were operated for 54 days. MEP were appeared successful to remove all phosphate concentration from the column influent having 2 mg/L concentration. The operated column reactors were successfully regenerated with alkaline solution. The results indicated potential for practical application of the MEP for phosphate removal.

Similar content being viewed by others
References
Almasri D A, Saleh N B, Atieh M A, McKay G, Ahzi S (2019). Adsorption of phosphate on iron oxide doped halloysite nanotubes. Scientific Reports, 9(1): 3232
Alorro R D, Hiroyoshi N, Kijitani H, Ito M, Tsunekawa M (2010). On the use of magnetite for gold recovery from chloride solution. Mineral Processing and Extractive Metallurgy Review, 31(4): 201–213
Alqadami A A, Naushad M, Abdalla M A, Ahamad T, Alothman Z A, Alshehri S M, Ghfar A A (2017). Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: A study of adsorption parameters and interaction mechanism. Journal of Cleaner Production, 156: 426–436
Asoufi H M, Al-Antary T M, Awwad A M (2018). Green route for synthesis hematite (α-Fe2O3) nanoparticles: Toxicity effect on the green peach aphid, Myzus persicae (Sulzer). Environmental Nano-technology, Monitoring & Management, 9: 107–111
Buzin PGWKD, Vigânico E M, Silva R D A, Heck N C, Schneider I A, Menezes J C S (2014). Prodution of ferrous sulfate from steelmeking mill scale. International Journal of Scientific and Engineering Research, 5(4): 353–359
Daou T J, Begin-Colin S, Grenèche J M, Thomas F, Derory A, Bernhardt P, Legaré P, Pourroy G (2007). Phosphate adsorption properties of magnetite-based nanoparticles. Chemistry of Materials, 19(18): 4494–4505
Funari V, Mantovani L, Vigliotti L, Tribaudino M, Dinelli E, Braga R (2018). Superparamagnetic iron oxides nanoparticles from municipal solid waste incinerators. Science of the Total Environment, 621: 687–696
Gan L, Lu Z, Cao D, Chen Z (2018). Effects of cetyltrimethylammonium bromide on the morphology of green synthesized Fe3O4 nanoparticles used to remove phosphate. Materials Science and Engineering C, 82: 41–45
Guaya D, Valderrama C, Farran A, Sauras T, Cortina J L (2018). Valorisation of N and P from waste water by using natural reactive hybrid sorbents: Nutrients (N,P,K) release evaluation in amended soils by dynamic experiments. Science of the Total Environment, 612: 728–738
Gypser S, Hirsch F, Schleicher A M, Freese D (2018). Impact of crystalline and amorphous iron- and aluminum hydroxides on mechanisms of phosphate adsorption and desorption. Journal of Environmental Sciences-China, 70: 175–189
Huang W, Zhang Y, Li D (2017). Adsorptive removal of phosphate from water using mesoporous materials: A review. Journal of Environmental Management, 193: 470–482
Islam M, Mishra S, Swain S K, Patel R, Dey R K, Naushad M (2014). Evaluation of phosphate removal efficiency from aqueous solution by polypyrrole/BOF slag nanocomposite. Separation Science and Technology, 49(17): 2668–2680
Khafri H Z, Ghaedi M, Asfaram A, Safarpoor M (2017). Synthesis and characterization of ZnS:Ni-NPs loaded on AC derived from apple tree wood and their applicability for the ultrasound assisted comparative adsorption of cationic dyes based on the experimental design. Ultrasonics Sonochemistry, 38: 371–380
Kumar A, Kumar A, Sharma G, Al-Muhtaseb A H, Naushad M, Ghfar A A, Stadler F J (2018). Quaternary magnetic BiOCl/g-C3N4/Cu2O/ Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment. Chemical Engineering Journal, 334: 462–478
Lee W H, Kim J O (2019). Effect of coexisting components on phosphate adsorption using magnetite particles in water. Environmental Science and Pollution Research International, 26(2): 1054–1060
Legodi M, Dewaal D (2007). The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes and Pigments, 74(1): 161–168
Li F, Wang L, Ji C, Wu H, Zhao J, Tang J (2017). Toxicological effects of tris(2-chloropropyl) phosphate in human hepatic cells. Chemosphere, 187: 88–96
Li Z, Qiu Z, Yang J, Ma B, Lu S, Qin C (2018). Investigation of phosphate adsorption from an aqueous solution using spent fluid catalytic cracking catalyst containing lanthanum. Frontiers of Environmental Science & Engineering, 12(6): 15
Mor S, Chhoden K, Ravindra K (2016). Application of agro-waste rice husk ash for the removal of phosphate from the wastewater. Journal of Cleaner Production, 129: 673–680
Ngo H H, Guo W (2009). Membrane fouling control and enhanced phosphorus removal in an aerated submerged membrane bioreactor using modified green bioflocculant. Bioresource Technology, 100(18): 4289–4291
Nohra J S A, Madramootoo C A, Hendershot W H (2007). Modelling phosphate adsorption to the soil: application of the non-ideal competitive adsorption model. Environmental Pollution, 149(1): 1–9
Pan B, Wu J, Pan B, Lv L, Zhang W, **ao L, Wang X, Tao X, Zheng S (2009). Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents. Water Research, 43(17): 4421–4429
Potapova E, Yang X, Westerstrand M, Grahn M, Holmgren A, Hedlund J (2012). Interfacial properties of natural magnetite particles compared with their synthetic analogue. Minerals Engineering, 36-38: 187–194
Pramanik M, Imura M, Lin J, Kim J, Kim J H, Yamauchi Y (2015). Shape-controlled synthesis of mesoporous iron phosphate materials with crystallized frameworks. Chemical Communications (Cambridge), 51(72): 13806–13809
Rashid M, Price N T, Gracia Pinilla M A, O’Shea K E (2017). Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Research, 123: 353–360
Raven K P, Jain A, Loeppert R H (1998). Arsenite and arsenate adsorption on ferrihydrite Kinetics, equilibrium, and adsorption envelopes. Environmental Science & Technology, 32(3): 344–349
Salazar-Camacho C, Villalobos M, Rivas-Sânchez M D L L, Arenas-Alatorre J, Alcaraz-Cienfuegos J, Gutiérrez-Ruiz M E (2013). Characterization and surface reactivity of natural and synthetic magnetites. Chemical Geology, 347: 233–245
Shahid M K, Choi Y G (2018). The comparative study for scale inhibition on surface of RO membranes in wastewater reclamation: CO2 purging versus three different antiscalants. Journal of Membrane Science, 546: 61–69
Shahid M K, Kim J Y, Choi Y G (2019a). Synthesis of bone char from cattle bones and its application for fluoride removal from the contaminated water. Groundwater for Sustainable Development, 8: 324–331
Shahid M K, Phearom S, Choi Y G (2018a). Synthesis of magnetite from raw mill scale and its application for arsenate adsorption from contaminated water. Chemosphere, 203: 90–95
Shahid M K, Phearom S, Choi Y G (2019b). Evaluation of arsenate adsorption efficiency of mill-scale derived magnetite particles with column and plug flow reactors. Journal of Water Process Engineering, 28: 260–268
Shahid M K, Pyo M, Choi Y G (2017). Carbonate scale reduction in reverse osmosis membrane by CO2 in wastewater reclamation. Membrane Water Treatment, 8(2): 125–136
Shahid M K, Pyo M, Choi Y G (2018b). The operation of reverse osmosis system with CO2 as a scale inhibitor: A study on operational behavior and membrane morphology. Desalination, 426: 11–20
Tofan-Lazar J, Al-Abadleh H A (2012). ATR-FTIR studies on the adsorption/desorption kinetics of dimethylarsinic acid on iron-(oxyhydr)oxides. Journal of Physical Chemistry A, 116(6): 1596–1604
Vikrant K, Kim K H, Ok Y S, Tsang D C W, Tsang Y F, Giri B S, Singh R S (2018). Engineered/designer biochar for the removal of phosphate in water and wastewater. Science of the Total Environment, 616-617:1242–1260
Wang L, Gao L (2011). Controlled synthesis and tunable properties of hematite hierarchical structures in a dual-surfactant system. Cryst-EngComm, 13(6): 1998–2005
Wang Z, Shen D, Shen F, Li T (2016). Phosphate adsorption on lanthanum loaded biochar. Chemosphere, 150: 1–7
**ao X, Liu S, Zhang X, Zheng S (2017). Phosphorus removal and recovery from secondary effluent in sewage treatment plant by magnetite mineral microparticles. Powder Technology, 306: 68–73
Yang B, Liu D, Lu J, Meng X, Sun Y (2018). Phosphate uptake behavior and mechanism analysis of facilely synthesized nanocrystalline Zn-Fe layered double hydroxide with chloride intercalation. Surface and Interface Analysis, 50(3): 378–392
Ye F, Li Y, Lin Q, Zhan Y (2017). Modeling of China’s cassava-based bioethanol supply chain operation and coordination. Energy, 120: 217–228
Yin M, Chen Z, Deegan B, O’brien S (2007). Wüstite nanocrystals: Synthesis, structure and superlattice formation. Journal of Materials Research, 22(7): 1987–1995
Yoon H S, Chung K W, Kim C J, Kim J H, Lee H S, Kim S J, Lee S I, Yoo S J, Lim B C (2018). Characteristics of phosphate adsorption on ferric hydroxide synthesized from a Fe2(SO4)3 aqueous solution discharged from a hydrometallurgical process. Korean Journal of Chemical Engineering, 35(2): 470–478
Yoon S Y, Lee C G, Park J A, Kim J H, Kim S B, Lee S H, Choi J W (2014). Kinetic, equilibrium and thermodynamic studies for phosphate adsorption to magnetic iron oxide nanoparticles. Chemical Engineering Journal, 236: 341–347
Zach-Maor A, Semiat R, Shemer H (2011). Adsorption-desorption mechanism of phosphate by immobilized nano-sized magnetite layer: Interface and bulk interactions. Journal of Colloid and Interface Science, 363(2): 608–614
Zhang L, Gao Y, Xu Y, Liu J (2016). Different performances and mechanisms of phosphate adsorption onto metal oxides and metal hydroxides: a comparative study. Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 91(5): 1232–1239
Zhang T, Xu H, Li H, He X, Shi Y, Kruse A (2018). Microwave digestion-assisted HFO/biochar adsorption to recover phosphorus from swine manure. Science of the Total Environment, 621: 1512–1526
Acknowledgements
This work was supported by 2015 Advanced Industrial Technology Development program by the Ministry of Environment (MOE), Republic of Korea (Project 2015000150006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• MEP were separated from mill scale at low magnetic intensity i.e., 300 to 500 gauss.
• The phosphate adsorption capacity of MEP was determined 6.41 mg/g.
• MEP packed-bed columns were successfully regenerated with alkaline solution.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Shahid, M.K., Kim, Y. & Choi, YG. Adsorption of phosphate on magnetite-enriched particles (MEP) separated from the mill scale. Front. Environ. Sci. Eng. 13, 71 (2019). https://doi.org/10.1007/s11783-019-1151-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-019-1151-2