Abstract
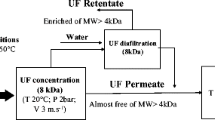
Rice brans were treated with a protease together with a disulfide bond-breaking agent (Na2SO3) to achieve a 2–4% peptide bond hydrolysis (DH). Ultrafiltration (UF) with 3 kDa molecular weight cut-off (MWCO) membrane led to substantial loss of feed protein due to permeation. Using 1 kDa MWCO membrane increased protein yields, but it was not effective in purifying the protein hydrolysates despite the increase in membrane area and operating time. The efficiency of this UF process can be improved using a larger MWCO membrane (e.g., 2 kDa MWCO), which may facilitate complete removal of phytate. Based on disparity of molecular sizes, use of phytase may also increase purity of protein retentates and allow the recovery of functional inositol phosphates in permeates. The presence of Na2SO3 during proteolysis to 2% DH of preheated bran (100°C, 10 min) repaired the damage caused by preheat treatment by increasing protein recovery but increased the concentration of small peptides in hydrolysates, i.e., <1 kDa, particularly for highly aggregated proteins. Heat treatment is necessary to stabilize rice bran, but the sulfite treatment may be avoided to increase UF yield and purity of protein retentates and allow higher DH values for hydrolysis of stabilized brans. Accordingly, this UF process can be an efficient method for recovering high-value components from rice bran, an underutilized rice milling co-product, for many industrial applications.
Similar content being viewed by others
References
Hamada, J.S., Characterization of Protein Fractions of Rice Bran to Devise Effective Methods of Protein Solubilization, Cereal Chem. 74:662–668 (1997).
Chen, L., and D.F. Houston, Solubilization and Recovery of Protein from Defatted Rice Bran, ——Ibid. 47:72–79 (1970).
Connor, M.A., R.M. Saunders, and G.O. Kohler, Preparation and Properties of Protein Concentrates Obtained by Wet Alkaline Processing of Rice Bran, in Proceedings of Rice By-Products Utilization, International Conference, 1974, Valencia, Spain, Vol. Iv, Rice Bran Utilization: Food and Feed, edited by S. Barber and E. Tortosa, Institute for Agricultural Chemistry and Food Technology, Valencia, 1977, pp. 189–202.
Lew, E.J.L., D.F. Houston, and D.A. Fellers, A Note on Protein Concentrate from Full-fat Rice Bran, Cereal Chem. 52:748–750 (1975).
Mitsuda, H., K. Yasumoto, and S. Yamamoto, Protein in Rice Bran and Polish for Human Nutrition, in Proceedings of Rice By-Products Utilization, International Conference, 1974, Valencia, Spain, Vol. Iv, Rice Bran Utilization: Food and Feed, edited by S. Barber and E. Tortosa, Institute for Agricultural Chemistry and Food Technology, Valencia, 1977, pp. 177–187.
Gnanasambandam, R., and N.S. Hettiarachchy, Protein Concentrates from Unstabilized and Stabilized Rice Bran: Preparation and Properties, J. Food Sci. 60:1066–1069, 1074 (1995).
Woodard, J.C., and D.D. Short, Toxicity of Alkali-Treated Soy Protein in Rats, J. Nutr. 103:569–574 (1973).
Hamada, J.S., Use of Proteases to Enhance Solubilization of Rice Bran Proteins, J. Food Biochem. 23:307–321 (1999).
Adler-Nissen, J., Enzymatic Hydrolysis of Food Proteins, Elsevier Applied Science Publishers, New York, 1986, pp. 263–331.
Hamada, J.S., Ultrafiltration for Recovery and Reuse of Peptidoglutaminase in Protein Deamidation, J. Food Sci. 56:1731–1734 (1991).
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall, Protein Measurement with Folin Phenol Reagent, J. Biol. Chem. 193:267–275 (1951).
Adler-Nissen, J., Determination of the Degree of Hydrolysis of Food Proteins by Trinitrobenzenesulfonic Acid, J. Agric. Food Chem. 27:1258–1262 (1979).
Hamada, J.S., and W.E. Marshall, Preparation and Functional Properties of Enzymatically Deamidated Soy Proteins, J. Food Sci. 54:598–601, 635 (1989).
Pearce, K.N., and J.E. Kinsella, Emulsifying Properties of Proteins: Evaluation of a Turbidimetric Technique, J. Agric. Food Chem. 26:716–723 (1978).
Circle, S.J., E.W. Meyer, and R.W. Whitney, Rheology of Soy Protein Dispersions. Effect of Heat and Other Factors on Gelation, Cereal Chem. 41:157–172 (1964).
Cheryan, M., Ultrafiltration and Microfiltration, Technomic Publishing Co., Inc., Lancaster, PA, 1998, pp. 345–494.
Puski, G., Modification of Functional Properties of Soy Proteins by Proteolytic Enzyme Treatment, Cereal Chem. 52:655–664 (1975).
Huang, Y.T., and J.E. Kinsella, Effects of Phosphorylation on Emulsifying and Foaming Properties and Digestibility of Yeast Protein, J. Food Sci. 52:1684–1688 (1987).
Barber, S., J. Camacho, R. Cerni, E. Tortosa, and E. Primo, Process for the Stabilization of Rice Bran. I. Basic Research Studies, in Proceedings of Rice By-Products Utilization, International Conference, 1974, Valencia, Spain, Vol. Ii By-Products Preservation, edited by S. Barber and E. Tortosa, Institute for Agricultural Chemistry and Food Technology, Valencia, 1977, pp. 49–62.
Barrientos, L., J.J. Scott, and P.P.N. Murthy, Specificity of Hydrolysis of Phytic Acid by Alkaline Phytase from Lily Pollen, Plant Physiol. 106:1489–1495 (1994).
Liu, H., Isolation and Identification of Phytases, Phytic Acid, and Inositol Phosphate from Rice Bran, M.S. Thesis, Department of Food Science, Lousiana State University, 1996.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hamada, J.S. Ultrafiltration of partially hydrolyzed rice bran protein to recover value-added products. J Amer Oil Chem Soc 77, 779–784 (2000). https://doi.org/10.1007/s11746-000-0124-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-000-0124-3